Team:Caltech/Week 7
From 2010.igem.org
(Difference between revisions)
| Line 4: | Line 4: | ||
==Monday 7/26== | ==Monday 7/26== | ||
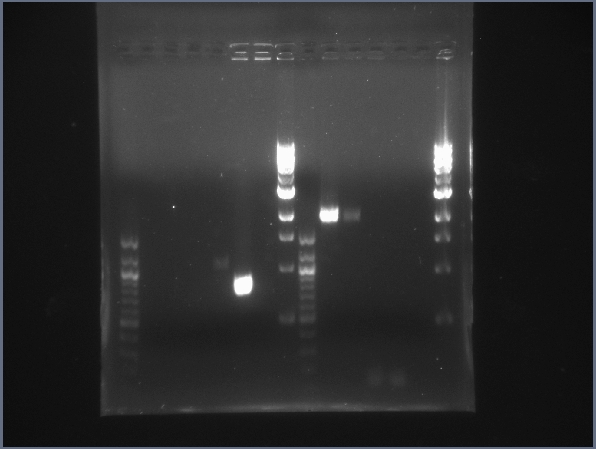
| + | [[Image:CIT-cpcr-gel1-7-26.jpg|200px|thumb|CPCR gel featuring L16, L19, L23, L24, L31 & L34. (7/26)]] | ||
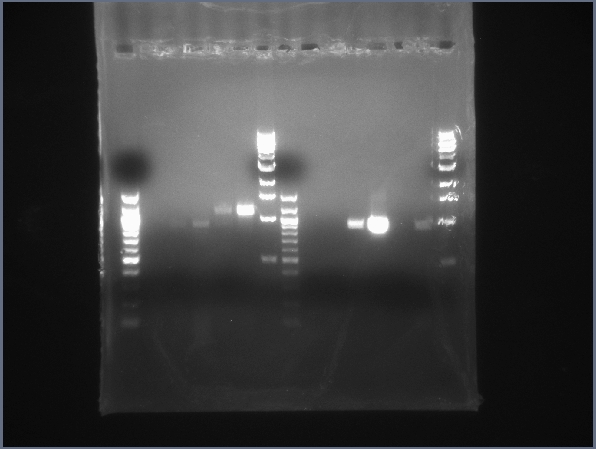
| + | [[Image:CIT-cpcr-gel2-7-26.jpg|200px|thumb|CPCR gel featuring L34, L35, L40, L42, L43 & L44. (7/26)]] | ||
* Started PCR reaction to extract the HSP (K112400) from its Berkeley Standard backbone (BBb). (Used same PCR parameters as CPCR from 7/22. | * Started PCR reaction to extract the HSP (K112400) from its Berkeley Standard backbone (BBb). (Used same PCR parameters as CPCR from 7/22. | ||
* Group meeting! | * Group meeting! | ||
| Line 26: | Line 28: | ||
* PCR purified K112400 PCR product & digested as plasmid insert. | * PCR purified K112400 PCR product & digested as plasmid insert. | ||
* Transformed RFP bricks of verious resistances to use as backbone for future ligations | * Transformed RFP bricks of verious resistances to use as backbone for future ligations | ||
| - | * Ran 2 gels of today's CPCR products | + | * Ran 2 gels of today's CPCR products. |
==Tuesday 7/27== | ==Tuesday 7/27== | ||
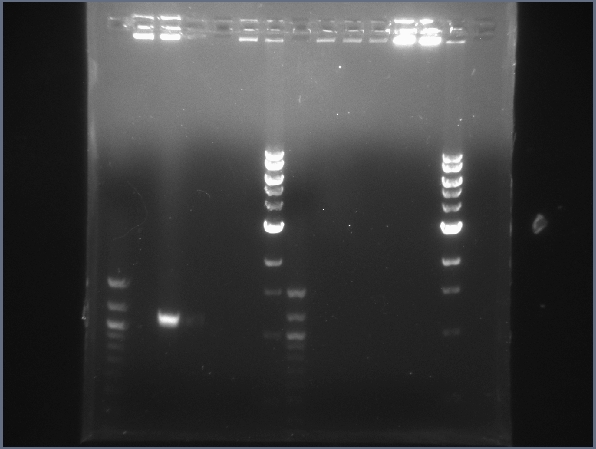
| + | [[Image:CIT-cpcr-gel-7-27.jpg|200px|thumb|CPCR gel featuring L16, L19, L31, L34, & L35. (7/22)]] | ||
* Began more CPCR on failed ones from yesterday: L16, L19, L31, L34, L35 | * Began more CPCR on failed ones from yesterday: L16, L19, L31, L34, L35 | ||
* Began more digests of miniprepped DNA from K112400 | * Began more digests of miniprepped DNA from K112400 | ||
Revision as of 19:04, 28 July 2010
|
People
|
Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | Week 11 | Week 12 | Week 13 | Week 14 | Week 15
Monday 7/26
Tuesday 7/27
Wednesday 7/28Thursday 7/29Friday 7/30Weekend 7/31-8/1
|
 "
"