Team:Panama/9 August 2010
From 2010.igem.org
(→August 9) |
(→August 9) |
||
| Line 2: | Line 2: | ||
==='''August 9'''=== | ==='''August 9'''=== | ||
| - | More electrophresis | + | '''More electrophresis''' |
| - | + | ||
High resolution agarose gel (for small fragments). In this electrophoresis we used two (1 and 5) for the molecular weight markers, the Lamda DNA/Hind III marker from Biotools. We decided to load duplicates of the samples, so we have the Promoter and RBS on lanes 2 & 3; lane 4 is empty and once again, the Promoter and RBS are in lanes 6 & 7. | High resolution agarose gel (for small fragments). In this electrophoresis we used two (1 and 5) for the molecular weight markers, the Lamda DNA/Hind III marker from Biotools. We decided to load duplicates of the samples, so we have the Promoter and RBS on lanes 2 & 3; lane 4 is empty and once again, the Promoter and RBS are in lanes 6 & 7. | ||
| Line 33: | Line 32: | ||
'''Restriction reaction Repetitions:''' | '''Restriction reaction Repetitions:''' | ||
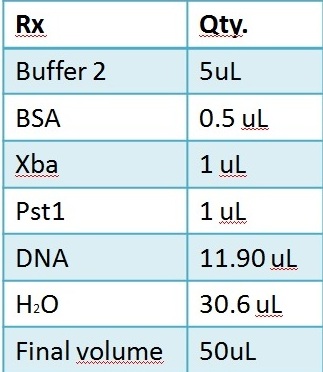
| - | 1. Promoter (using | + | 1. Promoter (using NEB restriction enzymes) |
[[Image:Untitled2.jpg|150px|thumb|left|alt text]] | [[Image:Untitled2.jpg|150px|thumb|left|alt text]] | ||
| - | 2. Promoter (using | + | 2. Promoter (using other restriction enzymes) |
[[Image:Untitled2.jpg|150px|thumb|left|alt text]] | [[Image:Untitled2.jpg|150px|thumb|left|alt text]] | ||
Revision as of 05:16, 22 October 2010
August 9
More electrophresis High resolution agarose gel (for small fragments). In this electrophoresis we used two (1 and 5) for the molecular weight markers, the Lamda DNA/Hind III marker from Biotools. We decided to load duplicates of the samples, so we have the Promoter and RBS on lanes 2 & 3; lane 4 is empty and once again, the Promoter and RBS are in lanes 6 & 7.
After the electrophoresis was done, we proceed to stain the gel with ethydium bromide: Ethydium Bromide staining:
The stock concentration was at 10mg/ml.
The concentration for gel staining was 0.5ug/ml.
We used 15ul of Ethydium Bromide in 300ml of dH2O. We stained it covered for 30 minutes. Afterwards, we washed it in water for 10 minutes each time.
Restriction reaction Repetitions:
1. Promoter (using NEB restriction enzymes)
2. Promoter (using other restriction enzymes)
3. RBS
The reason behind testing different batches of the same restriction enzymes for the promoter was to find out the reason why we didn't see anything when we ran electroforesis gels. We had thought that maybe the restriction enzymes were not doing their job, so we tried digesting the parts with enzymes that INDICASAT provided.
PsB1C3 Plasmid transformation (Stock):
- LB plates with Cloranfenicol at a concentration of 33ug/ml.
- LB plates with Tetracycline at a concentration of 15ug/ml.
|
|
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"