Team:Bielefeld-Germany/Project/Protocols
From 2010.igem.org
{{{1}}}
Contents |
Organisation and logistic
- flights in the US
- Discussion of possible Substances for detection
- list of Substances:
- 2-Chlorphenol (drug), Capcaicin (spiciness), Dopamin and its derivates (human hormones),
- 2,4,6 Trichloranisol(Responsible for bad taste in red wine))
- Literature research for the virA sensor system
- contact of reaserch groups in order to get an already working system (failed)
- Evaluation of the mutageneses strategy
- Error Proune PCR, DNA shuffling, directed evolution, Protein coupling assay
- contact to local newspapers; TV; Radio
Wetlab
accomplished
- qRT-PCR of induced aggrobacterium tumefaciens c58
- -> the strain could be significantly induced by acetosyringon
- Synthesis of the virG by MrGene (use without rpoA, clear of illegal restriction sites, codon usage for a.tumrfaciens)
- Testing of the Promega readout machine (GloMax multiplate reader) for the LUC-assay (working)
- -> calibration of the GloMax
- Testing of the virA construct from another researchgroup
- -> the virA construct could not be amplified
- Testing of the virA biobrick taken of the iGEM regestry
- -> Correction and improvement of the virA biobrick
- cloning of a constitutive promotor and a rbs in front of the improved virA
- cloning of virG into the right biobrick vector
- creating new antibiotic biobricks
- Testing Top10 and Ec100D for ccdb-gene
to be done
- characterisation of new build standalone virG biobrick
- characterisation of new build virA biobrick
- cloning of the construct backbone (promotor, virA, terminator, virB, virG, readout)
- Error Prone PCR of virA
- Sensitivity test by antibiotic gradient
- Modeling
- Constructing a new Backboneplasmid -> creating a cloning system in order to insert the r6k-origin in all psb-backbone plasmids
- Testing of the ccdb-death gene
Lab protocols
Transformation
- Thaw 150 µL competent E. coli cells on ice
- add max. 10 µL DNA (the less the better your transformation works but at least about 50 ng vector)
- incubate 30 min on ice
- heatshock: 42 °C, 45 s (water bath because of quick heat transfer)
- 1 min on ice
- add 1 mL prewarmed SOC-medium
- incubate: 45 - 60 min, 37 °C
- plate 100 µL
- spin down the remaining cells (2 min, 5000 g), discard most of the supernatant, resuspend the cells in the remaining medium and plate them
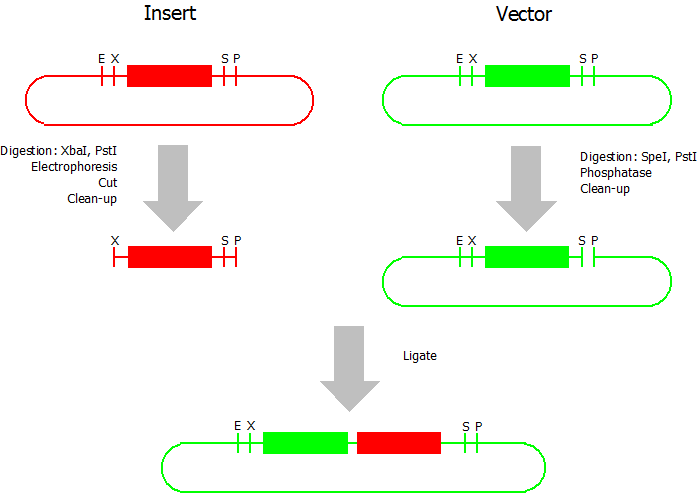
Silver BioBrick Assembly
This assembly method can be used for BioBricks which are bigger than 150 bp. The BioBrick should be at least 500 bp bigger or smaller than the backbone. The BioBrick, which complies with these conditions, is used as the insert and is assembled into the prefix or suffix of the other used BioBrick, called vector. So you have to differentiate between a prefix and a suffix insertion.
Suffix Insertion
- Digestion of insert: at least 700 ng DNA / 10 µL volume, 1 µL 10x Tango buffer, 0.5 µL XbaI, 1 µL PstI. Digest for 2 h at 37 °C, afterwards inactivation for 20 min at 80 °C. Clean up the insert via gel electrophoresis. When cutting the insert out of the gel try to avoid staining or exposure to ultraviolet light of the insert.
- Digestion of vector about 700 ng DNA / 10 µL volume, 1 µL 10x orange buffer, 0.5 µL SpeI, 0.5 µL PstI. Digest for 2h at 37 °C, afterwards inactivation for 20 min at 80 °C. Add 0.2 µL SAP (shrimp alcaline phosphatase), incubate for 1 h at 37 °C. Clean up the vector with a PCR clean-up kit.
- Ligation: after digestion and clean-up: 50 - 200 ng of vector, 3 - 10 fold molar access of insert, 20 µL ligation volume, 2 µL T4-Ligase-Buffer, 1 µL T4-Ligase. Incubate for 1 h at 37 °C, afterwards inactivation for 5 min at 70 °C. Then: store at -20 °C or transform.
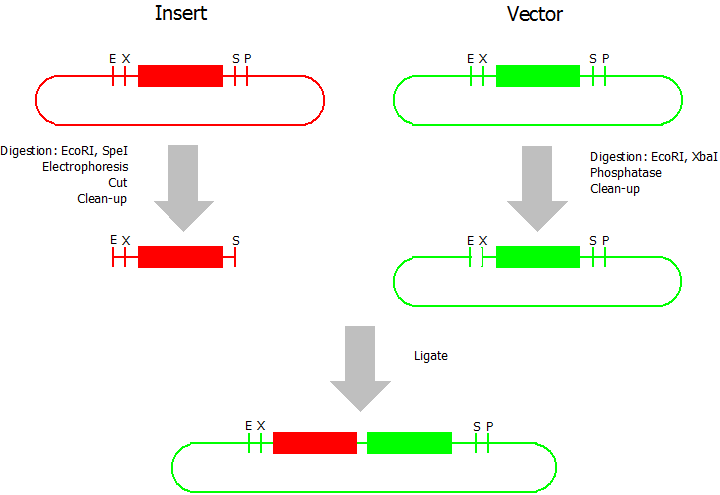
Prefix Insertion
- Digestion of insert: at least 700 ng DNA / 10 µL volume, 1 µL 10x BamHI buffer, 0.5 µL EcoRI, 0.5 µL SpeI. Digest for 2 h at 37 °C, afterwards inactivation for 20 min at 80 °C. Clean up the insert via gel electrophoresis. When cutting the insert out of the gel try to avoid staining or exposure to ultraviolet light of the insert.
- Digestion of vector about 700 ng DNA / 10 µL volume, 1 µL 10 x Tango buffer, 0.5 µL EcoRI, 0.5 µL XbaI. Digest for 2h at 37 °C, afterwards inactivation for 20 min at 80 °C. Add 0.2 µL SAP (shrimp alcaline phosphatase), incubate for 1 h at 37 °C. Clean up the vector with a PCR clean-up kit.
- Ligation: after digestion and clean-up: 50 - 200 ng of vector, 3 - 10 fold molar access of insert, 20 µL ligation volume, 2 µL T4-Ligase-Buffer, 1 µL T4-Ligase. Incubate for 1 h at 37 °C, afterwards inactivation for 5 min at 70 °C. Then: store at -20 °C or transform.
Variations
- A digestion over night is possible. If you digest over night use only 0.1 µL restriction enzyme.
- It is also possible to use PCR product as insert. Digest after PCR with corresponding restriction enzymes and clean up with PCR clean-up kit. This could lead to higher yields of insert DNA because a lot of DNA gets lost during the gel electrophoresis clean up.
Restriction analysis
- Digest BioBrick of interest: about 400 ng DNA / 10 µL volume, 1 µL 10x orange buffer, 0.5 µL NotI or PstI. Digest for 2 h at 37 °C. NotI is used to determine the length of the BioBrick and the plasmid backbone, PstI ist used to determine the length of the BioBrick in the plasmid backbone.
- Gel electrophoresis: add 2 µL loading buffer to every digestion mix, apply about 100 - 200 ng DNA / pocket in gel. Don't forget to apply the uncut BioBrick as well. A good agarose concentration for BioBricks between 0.2 and 3 kb is 1.5 %. The smaller your BioBrick of interest is the higher the agarose concentration should be and vice versa.
Chemicals, material etc.
| Chemical | Producer |
|---|---|
| Phusion polymerase | Finnzymes |
| Restriction enzymes | Fermentas |
| SAP | Fermentas |
| T4-Ligase | Fermentas |
 "
"