Team:Bielefeld-Germany/Project/Protocols
From 2010.igem.org
(Difference between revisions)
(→to be done) |
|||
| Line 73: | Line 73: | ||
* Testing of the ccdb-death gene | * Testing of the ccdb-death gene | ||
| + | |||
| + | |||
| + | === Lab protocols === | ||
| + | |||
| + | ==== Silver BioBrick Assembly ==== | ||
| + | |||
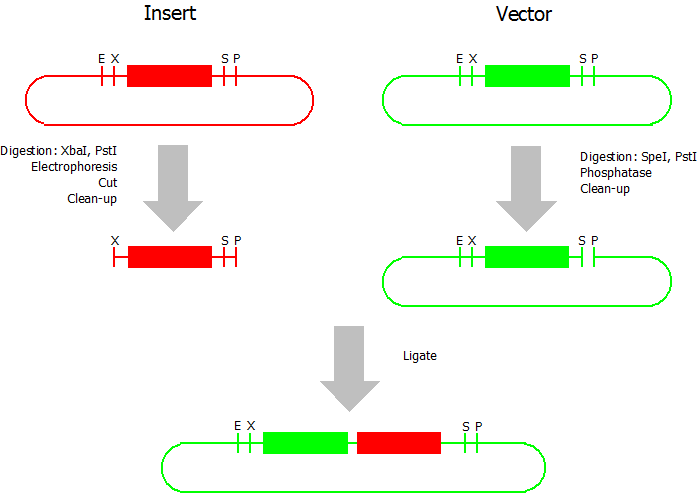
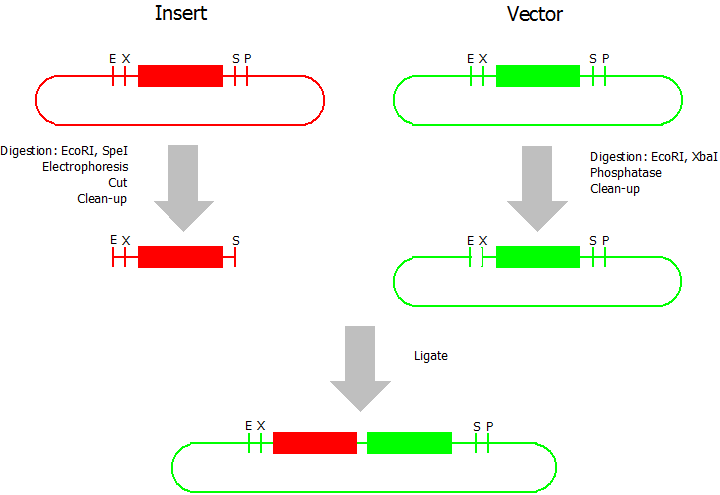
| + | This assembly method can be used for BioBricks which are bigger than 150 bp. The BioBrick should be at least 500 bp bigger or smaller than the backbone. The BioBrick, which complies with these conditions, is used as the insert and is assembled into the prefix or suffix of the other used BioBrick, called vector. So you have to differentiate between a prefix and a suffix insertion. | ||
| + | |||
| + | [[Image:Bielefeld_Silver_1.png|300px|thumb|right|Silver Suffix Insertion]] | ||
| + | [[Image:Bielefeld_Silver_2.png|300px|thumb|right|Silver Prefix Insertion]] | ||
| + | |||
| + | We used the following protocols. All enzymes and buffers were acquired by Fermentas and the clean-up kits by Machery-Nagel. | ||
| + | |||
| + | ===== Suffix Insertion ===== | ||
| + | |||
| + | * Digestion of insert: at least 700 ng DNA / 10 µL volume, 1 µL 10x Tango buffer, 0.5 µL XbaI, 1 µL PstI. Digest for 2 h at 37 °C, afterwards inactivation for 20 min at 80 °C. Clean up the insert via gel electrophoresis. | ||
| + | |||
| + | * Digestion of vector about 700 ng DNA / 10 µL volume, 1 µL 10x orange buffer, 0.5 µL SpeI, 0.5 µL PstI. Digest for 2h at 37 °C, afterwards inactivation for 20 min at 80 °C. Add 0.2 µL SAP (shrimp alcaline phosphatase), incubate for 1 h at 37 °C. Clean up the vector with a PCR clean-up kit. | ||
| + | |||
| + | * Ligation: after digestion and clean-up: 50 - 200 ng of vector, 3 - 10 fold molar access of insert, 20 µL ligation volume, 2 µL T4-Ligase-Buffer, 1 µL T4-Ligase. Incubate for 1 h at 37 °C, afterwards inactivation for 5 min at 70 °C. Then: store at -20 °C or transform. | ||
| + | |||
| + | |||
| + | ===== Prefix Insertion ===== | ||
| + | |||
| + | * Digestion of insert: at least 700 ng DNA / 10 µL volume, 1 µL 10x BamHI buffer, 0.5 µL EcoRI, 0.5 µL SpeI. Digest for 2 h at 37 °C, afterwards inactivation for 20 min at 80 °C. Clean up the insert via gel electrophoresis. | ||
| + | |||
| + | * Digestion of vector about 700 ng DNA / 10 µL volume, 1 µL 10 x Tango buffer, 0.5 µL EcoRI, 0.5 µL XbaI. Digest for 2h at 37 °C, afterwards inactivation for 20 min at 80 °C. Add 0.2 µL SAP (shrimp alcaline phosphatase), incubate for 1 h at 37 °C. Clean up the vector with a PCR clean-up kit. | ||
| + | |||
| + | * Ligation: after digestion and clean-up: 50 - 200 ng of vector, 3 - 10 fold molar access of insert, 20 µL ligation volume, 2 µL T4-Ligase-Buffer, 1 µL T4-Ligase. Incubate for 1 h at 37 °C, afterwards inactivation for 5 min at 70 °C. Then: store at -20 °C or transform. | ||
Revision as of 12:22, 27 August 2010
{{{1}}}
Contents |
Organisation and logistic
- flights in the US
- Discussion of possible Substances for detection
- list of Substances:
- 2-Chlorphenol (drug), Capcaicin (spiciness), Dopamin and its derivates (human hormones),
- 2,4,6 Trichloranisol(Responsible for bad taste in red wine))
- Literature research for the virA sensor system
- contact of reaserch groups in order to get an already working system (failed)
- Evaluation of the mutageneses strategy
- Error Proune PCR, DNA shuffling, directed evolution, Protein coupling assay
- contact to local newspapers; TV; Radio
Wetlab
accomplished
- qRT-PCR of induced aggrobacterium tumefaciens c58
- -> the strain could be significantly induced by acetosyringon
- Synthesis of the virG by MrGene (use without rpoA, clear of illegal restriction sites, codon usage for a.tumrfaciens)
- Testing of the Promega readout machine (GloMax multiplate reader) for the LUC-assay (working)
- -> calibration of the GloMax
- Testing of the virA construct from another researchgroup
- -> the virA construct could not be amplified
- Testing of the virA biobrick taken of the iGEM regestry
- -> Correction and improvement of the virA biobrick
- cloning of a constitutive promotor and a rbs in front of the improved virA
- cloning of virG into the right biobrick vector
- creating new antibiotic biobricks
- Testing Top10 and Ec100D for ccdb-gene
to be done
- characterisation of new build standalone virG biobrick
- characterisation of new build virA biobrick
- cloning of the construct backbone (promotor, virA, terminator, virB, virG, readout)
- Error Prone PCR of virA
- Sensitivity test by antibiotic gradient
- Modeling
- Constructing a new Backboneplasmid -> creating a cloning system in order to insert the r6k-origin in all psb-backbone plasmids
- Testing of the ccdb-death gene
Lab protocols
Silver BioBrick Assembly
This assembly method can be used for BioBricks which are bigger than 150 bp. The BioBrick should be at least 500 bp bigger or smaller than the backbone. The BioBrick, which complies with these conditions, is used as the insert and is assembled into the prefix or suffix of the other used BioBrick, called vector. So you have to differentiate between a prefix and a suffix insertion.
We used the following protocols. All enzymes and buffers were acquired by Fermentas and the clean-up kits by Machery-Nagel.
Suffix Insertion
- Digestion of insert: at least 700 ng DNA / 10 µL volume, 1 µL 10x Tango buffer, 0.5 µL XbaI, 1 µL PstI. Digest for 2 h at 37 °C, afterwards inactivation for 20 min at 80 °C. Clean up the insert via gel electrophoresis.
- Digestion of vector about 700 ng DNA / 10 µL volume, 1 µL 10x orange buffer, 0.5 µL SpeI, 0.5 µL PstI. Digest for 2h at 37 °C, afterwards inactivation for 20 min at 80 °C. Add 0.2 µL SAP (shrimp alcaline phosphatase), incubate for 1 h at 37 °C. Clean up the vector with a PCR clean-up kit.
- Ligation: after digestion and clean-up: 50 - 200 ng of vector, 3 - 10 fold molar access of insert, 20 µL ligation volume, 2 µL T4-Ligase-Buffer, 1 µL T4-Ligase. Incubate for 1 h at 37 °C, afterwards inactivation for 5 min at 70 °C. Then: store at -20 °C or transform.
Prefix Insertion
- Digestion of insert: at least 700 ng DNA / 10 µL volume, 1 µL 10x BamHI buffer, 0.5 µL EcoRI, 0.5 µL SpeI. Digest for 2 h at 37 °C, afterwards inactivation for 20 min at 80 °C. Clean up the insert via gel electrophoresis.
- Digestion of vector about 700 ng DNA / 10 µL volume, 1 µL 10 x Tango buffer, 0.5 µL EcoRI, 0.5 µL XbaI. Digest for 2h at 37 °C, afterwards inactivation for 20 min at 80 °C. Add 0.2 µL SAP (shrimp alcaline phosphatase), incubate for 1 h at 37 °C. Clean up the vector with a PCR clean-up kit.
- Ligation: after digestion and clean-up: 50 - 200 ng of vector, 3 - 10 fold molar access of insert, 20 µL ligation volume, 2 µL T4-Ligase-Buffer, 1 µL T4-Ligase. Incubate for 1 h at 37 °C, afterwards inactivation for 5 min at 70 °C. Then: store at -20 °C or transform.
 "
"