Team:Bielefeld-Germany/Project/Theory
From 2010.igem.org
(→VirA receptor structure) |
(→Phenolic Compounds) |
||
| Line 39: | Line 39: | ||
== Phenolic Compounds == | == Phenolic Compounds == | ||
| - | The Ligand receptor interaction between Acetosyringone and VirA is based on the interaction of several chemical groups. First of all, the hydroxylated aromat is essential. Metoxy groups in ortho position of the phenol play also crucial role in the signaling. Dimetoxy compounds have a higher activity than monometoxy compounds. The acetyl and alkyl groups in para position enhancing the binding affinity. VirA activating compounds must have two met-oxy groups in the ortho position and an additional carbonyl group on the R3 chain. The potential capacity of the group para to the phenolic hydroxyl group is associated with higher activities and chirality at this carbon center is critical for inducing activity | + | The Ligand receptor interaction between Acetosyringone and VirA is based on the interaction of several chemical groups. First of all, the hydroxylated aromat is essential. Metoxy groups in ortho position of the phenol play also crucial role in the signaling. Dimetoxy compounds have a higher activity than monometoxy compounds. The acetyl and alkyl groups in para position enhancing the binding affinity. VirA activating compounds must have two met-oxy groups in the ortho position and an additional carbonyl group on the R3 chain. The potential capacity of the group para to the phenolic hydroxyl group is associated with higher activities and chirality at this carbon center is critical for inducing activity (Yi-Han Lin ''et al.'', 2007), (McCullen CA., Binns AN. , 2006), (Winans SC., 1992,). |
| - | Regarding to the proton transfer model of Hess et al 1996 [26], the VirA activator transfers a proton to the basic area receptor binding site. The allosteric change leads to the phosphotransfer and the signaltransduction | + | Regarding to the proton transfer model of Hess et al 1996 [26], the VirA activator transfers a proton to the basic area receptor binding site. The allosteric change leads to the phosphotransfer and the signaltransduction (Yi Han Linn ''et al.'', 2008), (Kyunghee Lee, 1996). |
== Inducing enhancers == | == Inducing enhancers == | ||
Revision as of 14:45, 25 October 2010
Contents |
Introduction
Our iGEM project is to create an E. coli cell which is capable to sense Capsaicin in a complex sample and report the concentration with a luciferase light signal. The native receptor senses Acetosyringone. We used directed evolution to modify the binding region of the native receptor to generate a new capsaicin receptor. We established a screening system based on antibiotic concentration gradients to screen the newly generated receptors. In our E. coli Acetosyringone sensing system several parts driving from different organisms were assemlbed. The native receptor was subcloned from Agrobacterium tumefaciens to create a read out system with firefly luciferase.
Agrobacterium tumefaciens
The model organism Agrobacterium tumefaciens is a soil bacterium and can be found at nearly every point of the world Agrobacterium became known as a phyto-pathogen leading to the crown gall disease in dicotyledonous species( DeCleene M, DeLay J, 1976). The infection is actually caused by a gene transfer system located on an extrachromosomal element, the Ti plasmid. The infection can be divided into several steps. Predominantely A. tumefaciens senses phenolic compunds from hurted plants, but also aldose monosaccharides, low pH and low phosphate (Palmer AG. et al.2004),(Brencic A, Winans SC, 2005). When A. tumefaciens recognizes phenols with the virA receptor, a signal transduction cascade is initiated leadig to the expression of virulence genes. The next step is a physical interaction with the host plant. A type three secretion system is responsible for the DNA transfer of the Ti-plasmid from the bacterium into the host. The DNA is translocated to the nucleus, leading to the gene expression and the production of opin. A. tumefaciens uses the reprogrammed plant cells for metabolite production and therefore as a nutrient supplier.
For biotechnology purpose the Ti-Plasmid was disharmed. Instead the native transfer region (T-region) and a gene of interest could be easily introduced into the Ti plasmid. Agrobacterium-mediated DNA transfer is one of the most commonly used techniques of plant transformation (Ziemienowicz A, 2001).
Native receptor
To gain an evolutionary advance, A. tumefaciens needs a precise recognition system for potential hosts. The native sensing system is a two-component phospho-relay system in which VirA is an transmembrane-bound sensor while VirG is the intracellular response regulator (Wolanin PM et al., 2002). The two genes for the sensing system are virA and virG (Stachel SE, Nester EW, 1986) which are constitutively expressed at a basal level. VirA is a hitstidine kinase and after sensing the phenol 3,5-Dimethoxyacetophenone, Acetosyringone an autophosphorylation occurs at the His-474 residue (Huang Y et al., 1990),(Jin S et al., 1990). Later in the signal transduction cascade, the phosphorylated VirA leads to the transfer of the phosphate to Asp-52 residue of VirG (Jin SG, Prusti RK et al., 1990), (Jin SG, Roitsch T et al., 1990), (Pazour GJ, Das A, 1990). VirG is the response regulator of the two-component system (Brencic A, Winans SC, 2005). VirG acts as a transcription factor and binds to virulence box (vir Box) containing promotors, for example virB (] Jin SG, Roitsch T et al., 1990), (Pazour GJ, Das A, 1990)
VirA receptor structure
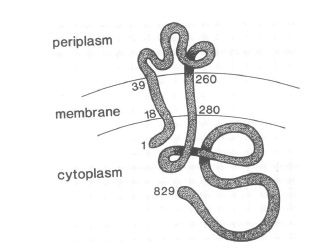
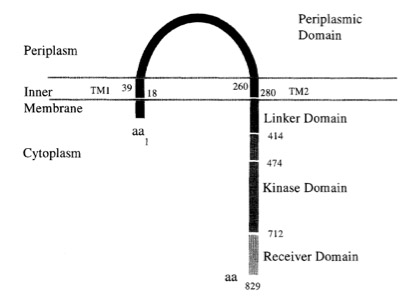
The virA receptor consists of 829 amino acids and is a transmembrane protein in the inner menbrane of A.tumefaciens [14]. VirA spans the inner membrane, with two transmembrane domains, a large periplasmic region, and a large C-terminal cytoplasmic domain. [16]. VirA directly senses the phenolic compounds for vir activation [15]. Therefore the linker domain is essential for induction by phenolic compounds [17]. The linker region is located in the cytosolic site at position 280 to 414 [15]. This region between aa 283 and 304 was highly conserved in four different strains of Agrobacterium, and therefore likely to serve as the receptor region for the phenolic inducers which are common to all four strains [18].
- Picture space holder
Chang and Winnans revealed in their studies, the parts of the VirA receptor essential for the signal transduction (] Chang CH, Winans SC., 1992). A structured model for different inducing conditions are shown in the figure below.
Phenolic Compounds
The Ligand receptor interaction between Acetosyringone and VirA is based on the interaction of several chemical groups. First of all, the hydroxylated aromat is essential. Metoxy groups in ortho position of the phenol play also crucial role in the signaling. Dimetoxy compounds have a higher activity than monometoxy compounds. The acetyl and alkyl groups in para position enhancing the binding affinity. VirA activating compounds must have two met-oxy groups in the ortho position and an additional carbonyl group on the R3 chain. The potential capacity of the group para to the phenolic hydroxyl group is associated with higher activities and chirality at this carbon center is critical for inducing activity (Yi-Han Lin et al., 2007), (McCullen CA., Binns AN. , 2006), (Winans SC., 1992,). Regarding to the proton transfer model of Hess et al 1996 [26], the VirA activator transfers a proton to the basic area receptor binding site. The allosteric change leads to the phosphotransfer and the signaltransduction (Yi Han Linn et al., 2008), (Kyunghee Lee, 1996).
Inducing enhancers
The sensitivity of this system is highly enhanced when additional aldose monosacchardic suggars occur in the environment of Agrobacterium. The sugar binding protein ChvE interact with the VirA receptor, leading to a much stronger vir gene expression [27].
Subcloning into E.coli and receptor function in new host
In our project we decided to work with E coli instead of A. tumefaciens. The transcription procedure in E. coli is very similar to A. tumefaciens but not complete homolog. In E. coli the rpoA gene - encoding the α-subunit of RNA polymerase in A. tumefaciens is not present but essential for the transcription of a virB promoter driven genen [29]. For this reason it was necessary to subclone a modified virG gene that is capaple to be trancribed by the E. coli expression system.
Yong-Chul described VirG conderived mutants that are capable of expressing the virB promoter-driven gene in E. coli without the requirement for the RpoA from A. tumefaciens, suggesting that the virG mutants are able to interact with the transcription system of E.coli [28]. In VirG the amino acid at position 56 is likely to play a key role in the interaction with the RpoA of E. coli. Regarding to [28 we used virG mutants, with amino acid substitutions of G56V and I77V that are capable of activating vir genes in E. coli in response to inducer acetosyringone in a virA-dependent manner.
Read out system
Receptor modification strategy
Random mutagenesis by error-prone PCR (EP-PCR)
In order to detect novel substances (e.g. capsaicin) with the virA receptor, the first step was to create a mutagenised library of virA variants, which could subsequently be screened for new binding characteristics. Plenty of different strategies for mutagenesis of DNA are known, including the use nucleotide analogues, bacteria containing mutator genes, the mutagenesis with UV light or chemicals and inaccurate PCR (Cadwell and Joyce, 1992).
When designing our strategy we rejected the use of bacteria strains with high mutation rates, since the changes in base sequence would occur all over the transformed plasmids. Thereby some mutations would also take place in the backbone of the plasmid, our might even been found in the standardized BB prefix and suffix. We also excluded the possibility of using UV light or mutagenic chemicals, due to reasons of safety and minimizing the exposure toxic substances.
In our experiment we wanted to alter only the part of the plasmid coding for the virA receptor, while using a not harmful and thereby safe technique. Thus, our method of choice was inaccurate PCR that allows the exclusive variations of a distinct region of a plasmid, which is defined by the location of the upstream and downstream primers. This mutagenic method of PCR, called error-prone PCR (EP-PCR) has been described and improved a lot in scientific community (McCullum et al., 2010).
The basic principle of this technique uses the natural high infidelity of the Taq DNA polymerase, which can even be increased by special changes in buffer conditions compared to standard PCR. These alterations may include the unequal distribution of dNTPs (5 mM purines, 25 mM pyrimidines) as well as an increased amount of MgCl2 and the addition of MnCl2. The total rate of base exchange can be adjusted by the number of PCR cycles, since mutations will accumulate during the exponential amplification of the sequence (Wilson and Keefe, 2000). The experimental conditions of the performed error-prone PCR are described in the section “protocols”.
Screening system
Modeling
Weblinks
[1] DeCleene M, DeLay J, 1976, The host range of crown gall, Bot Rev 42: 389–466
[2] Palmer AG, Gao R, Maresh J, Erbil WK, Lynn DG, 2004 Chemical biology of multi-host/pathogen interactions: chemical perception and metabolic complementation, Annu Rev Phytopathol 42: 439–464
[3] Brencic A, Winans SC, 2005, Detection of and response to signals involved in host-microbe interactions by plant-associated bacteria., Microbiol Mol Biol Rev 69: 155–194
[4] Wolanin PM, Thomason PA, Stock J.B., 2002, Histidine protein kinases: key signal transducers outside the animal kingdom., Genome Biol 3: REVIEWS3013
[5] Stachel SE, Nester EW, 1986, The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens., EMBO J 5: 1445–1454
[6] Ziemienowicz A., 2001, Odyssey of agrobacterium T-DNA,. Acta Biochim Pol. 2001;48(3):623-35.
[7] Jin SG, Roitsch T, Christie PJ, Nester EW, 1990, The regulatory VirG protein specifically binds to a cis-acting regulatory sequence involved in transcriptional activation of Agrobacterium tumefaciens virulence genes., J Bacteriol 172:531–537
[8] Pazour GJ, Das A, 1990, Characterization of the VirG binding site of Agrobacterium tumefaciens., Nucleic Acids Res 18:6909–6913
[9] Huang Y, Morel P, Powell B, Cado CI, 1990, VirA, a corregulator of Ti-specific virulence genes, is phosphorylated in vitro., J Bacteriol 172:1142–1144
[10] Jin S, Roitsch T, Ankenbauer RG, Gordon MP, Nester EW, 1990, The VirA protein of Agrobacterium tumefaciens is autophosphorylated and is essential for vir gene regulation., J Bacteriol 172: 525–530
[11] Jin SG, Prusti RK, Roitsch T, Ankenbauer RG, Nester EW, 1990, Phosphorylation of the VirG protein of Agrobacterium tumefaciens by the autophosphorylated VirA protein: Essential role in biological activity of VirG., J Bacteriol 172:4945–4950
[12] Jin SG, Roitsch T, Christie PJ, Nester EW, 1990, The regulatory VirG protein specifically binds to a cis-acting regulatory sequence involved in transcriptional activation of Agrobacterium tumefaciens virulence genes., J Bacteriol 172:531–537
[13] Pazour GJ, Das A, 1990, virG, an Agrobacterium tumefaciens transcriptional activator, initiates translation at a UUG codon and is a sequence-specific DNA-binding protein., J Bacteriol 172:1241– 1249
[14] Melchers LS, 1989, Membrane topology and functional analysis of the sensory protein VirA of Agrobacterium tumefaciens., The EMBO Journal vol.8 no.7 pp.1919- 1925
[15] Lee YW, Jin S, Sim WS, Nester EW, 1996, The sensing of plant signal molecules by Agrobacterium: genetic evidence for direct recognition of phenolic inducers by the VirA protein., Gene. 179(1):83-8.
[16] Banta LM, Joerger RD, Howitz VR, Campbell AM, Binns AN., 1994, Glu-255 outside the predicted ChvE binding site in VirA is crucial for sugar enhancement of acetosyringone perception by Agrobacterium tumefaciens., J Bacteriol. 176(11):3242-9.
[17] Chang CH, Winans SC., 1992, Functional roles assigned to the periplasmic, linker, and receiver domains of the Agrobacterium tumefaciens VirA protein., J Bacteriol. 174(21):7033-9.
[18] Turk SC, van Lange RP, Regensburg-Tuïnk TJ, Hooykaas PJ., 1994, Localization of the VirA domain involved in acetosyringone-mediated vir gene induction in Agrobacterium tumefaciens., Plant Mol Biol. 25(5):899-907.
[19] SCOTT M. LOHRKE, 2001, Reconstitution of Acetosyringone-Mediated Agrobacterium tumefaciens Virulence Gene Expression in the Heterologous Host Escherichia coli ., J. of Bacteriology, p. 3704–3711 Vol. 183, No. 12
[20] Winans SC., 1992, Two-way chemical signaling in Agrobacterium-plant interactions., Microbiol Rev. 56(1):12-31.
[21] McCullen CA., Binns AN. , 2006, Agrobacterium tumefaciens and Plant Cell Interactions and Activities Required for Interkingdom Macromolecular Transfer, Annu. Rev. Cell Dev. Biol. 22:101-127
[22] Yi-Han Lin et al., 2007, The initial steps in Agrobacterium tumefaciens pathogenesis: chemical biology of host recognition
[23]
[24] Yi Han Linn et al., 2008, Capturing the VirA/VirG TCS of Agrobacterium tumefaciens., Adv Exp Med Biol. 631:161-77.
[25] Kyunghee Lee, 1996, A structure-based activation model of phenol-receptor protein interactions.
[26] Hess KM, Dudley MW, Lynn DG, Joerger RD, Binns AN., 1991, Mechanism of phenolic activation of Agrobacterium virulence genes: development of a specific inhibitor of bacterial sensor/response systems., Proc. Natl. Acad. Sci. USA 88:7854–58
[27] Shimoda N,Toyoda-Yamamoto A, Shinsuke S, Machica Y., 1993, Genetic evidence for an interaction between the VirA sensor protein and the ChvE sugar-binding protein of Agrobacterium. J. Biol. Chem. 268:26552–58
[28] Yong-Chul J., Yunrong G., Donghai W., Shouguang J., 2004, Mutants of Agrobacterium tumefaciens virG Gene That Activate Transcription of vir Promoter in Escherichia coli Current Microbiology Vol. 49, pp. 334–340
[29] Lohrke SM, Nechaev S, Yang H, Severinov K, Jin SJ, 1999, Transcriptional activation of Agrobacterium tumefaciens virulence gene promoters in Escherichia coli requires the A. tumefaciens RpoA gene, encoding the alpha subunit of RNA polymerase., J Bacteriol 181:4533–4539
Literatur zur error-prone PCR
McCullum EO, Willliams BAR, Zhang J, Chaput JC (2010) Random Mutagenesis by Error-Prone PCR, In vitro mutagenesis protocols, Methods in Molecular Biology, Vol 634, 103-109.
Cadwell RC and Joyce GF (1992) Randomization of genes by PCR mutagenesis, Genome Res., 28-33.
Wilson DS, Keefe AD (2000) Random Mutagenesis by PCR, Current Protocols in Molecular Biology, 8.3.1-8.3.9.
 "
"