Team:Bielefeld-Germany/Project/Protocols
From 2010.igem.org
(→Transformation) |
|||
| Line 3: | Line 3: | ||
= Lab protocols = | = Lab protocols = | ||
| - | == Transformation == | + | |
| + | == Preparation of electrocompetent E.coli cells == | ||
| + | |||
| + | modified from Quint Lab | ||
| + | http://openwetware.org/wiki/Quint_Lab:electrocompetent_cells_e.coli | ||
| + | this protocol works fine with E.coli DB3.1 cells | ||
| + | |||
| + | |||
| + | Materials: | ||
| + | • 10 mL LB-Medium (1% Bacto Trypton; 0,5 % Yeast Extract; 0,5% NaCl) | ||
| + | • 2L cooled bidest H2O | ||
| + | • 200 mL cooled, sterile-filtered 10% Glycerol | ||
| + | • box with ice-water for 2-litre-flask | ||
| + | • 4 pre-cooled 250 mL (or 2x500 mL) bins for centrifugation | ||
| + | • 2 pre-cooled 50 mL Falcons | ||
| + | • centrifuge pre-cooled to 2°C (max. 4°C) | ||
| + | |||
| + | Method | ||
| + | * inoculate 2x10 mL LB with bacterial stock; incubate over night at 37°C and 200 rpm | ||
| + | * inoculate 2x250 mL LB in 1-litre-flask with OD600=0,1 @37°C or @ 19°C over night | ||
| + | * incubate until OD600 0,4-0,6 (~5 h) | ||
| + | * from now on everything is done at 2-4°C (best in a cold room) | ||
| + | |||
| + | * cool 1L-culture 10-15 minutes in ice water (shake sometimes) | ||
| + | * divide culture into 2x5 cooled 50 mL Falcon-tubes for centrifugation | ||
| + | * centrifuge 10min @ 4°C, 4000 rcf | ||
| + | * discard supernatant | ||
| + | * resuspend pellet in 5 mL bidest H2O | ||
| + | * add bidest H2O up to 50 mL | ||
| + | * centrifuge 10min @ 4°C, 4000 rcf | ||
| + | * discard supernatant immediately | ||
| + | * resuspend pellet in residual supernatant | ||
| + | * add bidest H2O up to 50 mL | ||
| + | * centrifuge 10min @ 4°C, 4000 rcf | ||
| + | * discard supernatant immediately | ||
| + | * resuspend pellet in residual supernatant | ||
| + | * transfer suspension in 2x50 mL Falcons | ||
| + | * add 10% Glycerol up to 50 mL | ||
| + | * centrifuge 5 min @ 4°C, 4000 rcf | ||
| + | * discard supernatant | ||
| + | * estimate volume of the pellet; fill up with equal volume of 10% Glycerol | ||
| + | * resuspend pellet on ice; ‘’’don´t vortex!!’’’ (just shake cautiously) | ||
| + | * divide cells into 150 µL Alicuots (use 1,5 mL Eppis) | ||
| + | * freeze in liquid N2 or dry-ice | ||
| + | * store @ -80°C | ||
| + | |||
| + | |||
| + | == Transformation via electroporation == | ||
| + | |||
| + | * add 0,5-2 µL plasmid to 50 µl electrocompetent cells | ||
| + | * electroporate at U=2,5 kV, C= 25 µF, R = 200 Ώ | ||
| + | * transfer transformation reaction to 450 µL SOC-Medium and shake 1 h at 37°C | ||
| + | * centrifuge 2 min @ 800 rpm and plate on selective LB-Medium | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | == Heat shock transformation == | ||
* Thaw 150 µL competent ''E. coli'' cells on ice | * Thaw 150 µL competent ''E. coli'' cells on ice | ||
Revision as of 15:36, 2 October 2010
Contents |
Lab protocols
Preparation of electrocompetent E.coli cells
modified from Quint Lab http://openwetware.org/wiki/Quint_Lab:electrocompetent_cells_e.coli this protocol works fine with E.coli DB3.1 cells
Materials:
• 10 mL LB-Medium (1% Bacto Trypton; 0,5 % Yeast Extract; 0,5% NaCl)
• 2L cooled bidest H2O
• 200 mL cooled, sterile-filtered 10% Glycerol
• box with ice-water for 2-litre-flask
• 4 pre-cooled 250 mL (or 2x500 mL) bins for centrifugation
• 2 pre-cooled 50 mL Falcons
• centrifuge pre-cooled to 2°C (max. 4°C)
Method
- inoculate 2x10 mL LB with bacterial stock; incubate over night at 37°C and 200 rpm
- inoculate 2x250 mL LB in 1-litre-flask with OD600=0,1 @37°C or @ 19°C over night
- incubate until OD600 0,4-0,6 (~5 h)
- from now on everything is done at 2-4°C (best in a cold room)
- cool 1L-culture 10-15 minutes in ice water (shake sometimes)
- divide culture into 2x5 cooled 50 mL Falcon-tubes for centrifugation
- centrifuge 10min @ 4°C, 4000 rcf
- discard supernatant
- resuspend pellet in 5 mL bidest H2O
- add bidest H2O up to 50 mL
- centrifuge 10min @ 4°C, 4000 rcf
- discard supernatant immediately
- resuspend pellet in residual supernatant
- add bidest H2O up to 50 mL
- centrifuge 10min @ 4°C, 4000 rcf
- discard supernatant immediately
- resuspend pellet in residual supernatant
- transfer suspension in 2x50 mL Falcons
- add 10% Glycerol up to 50 mL
- centrifuge 5 min @ 4°C, 4000 rcf
- discard supernatant
- estimate volume of the pellet; fill up with equal volume of 10% Glycerol
- resuspend pellet on ice; ‘’’don´t vortex!!’’’ (just shake cautiously)
- divide cells into 150 µL Alicuots (use 1,5 mL Eppis)
- freeze in liquid N2 or dry-ice
- store @ -80°C
Transformation via electroporation
- add 0,5-2 µL plasmid to 50 µl electrocompetent cells
- electroporate at U=2,5 kV, C= 25 µF, R = 200 Ώ
- transfer transformation reaction to 450 µL SOC-Medium and shake 1 h at 37°C
- centrifuge 2 min @ 800 rpm and plate on selective LB-Medium
Heat shock transformation
- Thaw 150 µL competent E. coli cells on ice
- add max. 10 µL DNA (the less the better your transformation works but at least about 50 ng vector)
- incubate 30 min on ice
- heatshock: 42 °C, 45 s (water bath because of quick heat transfer)
- 1 min on ice
- add 1 mL prewarmed SOC-medium
- incubate: 45 - 60 min, 37 °C
- plate 100 µL
- spin down the remaining cells (2 min, 5000 g), discard most of the supernatant, resuspend the cells in the remaining medium and plate them
Silver BioBrick Assembly
This assembly method can be used for BioBricks which are bigger than 150 bp. The BioBrick should be at least 500 bp bigger or smaller than the backbone. The BioBrick, which complies with these conditions, is used as the insert and is assembled into the prefix or suffix of the other used BioBrick, called vector. So you have to differentiate between a prefix and a suffix insertion.
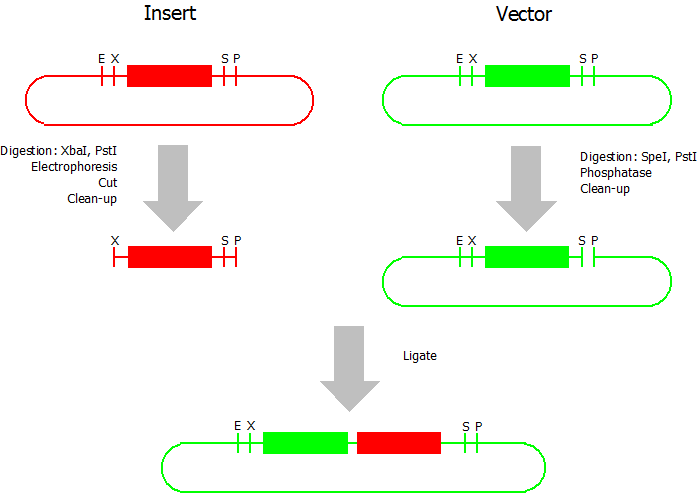
Suffix Insertion
- Digestion of insert: at least 700 ng DNA / 10 µL volume, 1 µL 10x Tango buffer, 0.5 µL XbaI, 1 µL PstI. Digest for 2 h at 37 °C, afterwards inactivation for 20 min at 80 °C. Clean up the insert via gel electrophoresis. When cutting the insert out of the gel try to avoid staining or exposure to ultraviolet light of the insert.
- Digestion of vector about 700 ng DNA / 10 µL volume, 1 µL 10x orange buffer, 0.5 µL SpeI, 0.5 µL PstI. Digest for 2h at 37 °C, afterwards inactivation for 20 min at 80 °C. Add 1 µL SAP (shrimp alcaline phosphatase) and 1.2 µL 10 x SAP buffer, incubate for 1 h at 37 °C. Clean up the vector with a PCR clean-up kit.
- Ligation: after digestion and clean-up: 50 - 200 ng of vector, 3 - 10 fold molar access of insert, 20 µL ligation volume, 2 µL T4-Ligase-Buffer, 1 µL T4-Ligase. Incubate for 1 h at 37 °C, afterwards inactivation for 5 min at 70 °C. Then: store at -20 °C or transform.
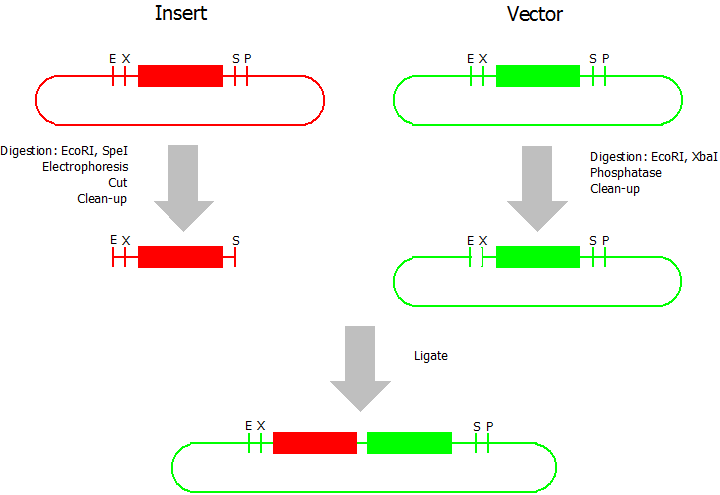
Prefix Insertion
- Digestion of insert: at least 700 ng DNA / 10 µL volume, 1 µL 10x BamHI buffer, 0.5 µL EcoRI, 0.5 µL SpeI. Digest for 2 h at 37 °C, afterwards inactivation for 20 min at 80 °C. Clean up the insert via gel electrophoresis. When cutting the insert out of the gel try to avoid staining or exposure to ultraviolet light of the insert.
- Digestion of vector about 700 ng DNA / 10 µL volume, 1 µL 10 x Tango buffer, 0.5 µL EcoRI, 0.5 µL XbaI. Digest for 2h at 37 °C, afterwards inactivation for 20 min at 80 °C. Add 1 µL SAP (shrimp alcaline phosphatase) and 1.2 µL 10 x SAP buffer, incubate for 1 h at 37 °C. Clean up the vector with a PCR clean-up kit.
- Ligation: after digestion and clean-up: 50 - 200 ng of vector, 3 - 10 fold molar access of insert, 20 µL ligation volume, 2 µL T4-Ligase-Buffer, 1 µL T4-Ligase. Incubate for 1 h at 37 °C, afterwards inactivation for 5 min at 70 °C. Then: store at -20 °C or transform.
Variations
- A digestion over night is possible. If you digest over night use only 0.1 µL restriction enzyme.
- It is also possible to use PCR product as insert. Digest after PCR with corresponding restriction enzymes and clean up with PCR clean-up kit. This could lead to higher yields of insert DNA because a lot of DNA gets lost during the gel electrophoresis clean up.
- Sometimes some BioBricks are hard to assemble. Then you have to clean up the vector by gel electrophoresis as well.
Colony PCR
- pick one colony with a sterile tip and elute it in 100 µl ddH20 or medium
- store the colony in 4°C while colony PCR is running
- one reaction mix contains:
- 2.5 µl 10x buffer
- 0.75 µl MgCl2
- 1 µl dNTPs
- 0.5 µl primer mix (prefix/suffix primers or sequencing primers)
- 19.25 µl ddH2O
- 0.5 µl taq-polymerase
- 0.5 µl template
- PCR program:
- start: 8 min, 98 °C
- 30 cycles of:
- 30 s, 98 °C
- 30 s, 60 °C
- 30 s / 1 kb template, 72 °C
- finish: 5 min, 72 °C
- gel electrophoresis: check the fragment size
- plate the correct colony
Restriction analysis
- Digest BioBrick of interest: about 400 ng DNA / 10 µL volume, 1 µL 10x orange buffer, 0.5 µL NotI or PstI. Digest for 2 h at 37 °C. NotI is used to determine the length of the BioBrick and the plasmid backbone, PstI ist used to determine the length of the BioBrick in the plasmid backbone.
- Gel electrophoresis: add 2 µL loading buffer to every digestion mix, apply about 100 - 200 ng DNA / pocket in gel. Don't forget to apply the uncut BioBrick as well. A good agarose concentration for BioBricks between 0.2 and 3 kb is 1.5 %. The smaller your BioBrick of interest is the higher the agarose concentration should be and vice versa. The gel electrophoresis is made with TAE-buffer. Be sure that you melt your agarose gel in the same buffer you use for the electrophoresis later.
Chemicals, material etc.
| Enzyme | Producer |
|---|---|
| Phusion polymerase | Finnzymes |
| Restriction enzymes | Fermentas |
| SAP | Fermentas |
| T4-Ligase | Fermentas |
TAE buffer
For 1 L of 50 x TAE buffer you need:
- 242.48 g Tris
- 41.02 g Sodiumacetate
- 18.612 g EDTA
- adjust pH to 7.8
- solve in dH2O
20 mL of the stock is diluted in 1 L dH2O for the gel electrophoresis.
DNA loading buffer
- 50 % (v/v) Glycerine
- 1 mM EDTA
- 0.1 % (w/v) Bromphenol blue
- solve in ddH20
LB medium
For 1 L of LB medium you need:
- 10 g Trypton
- 5 g yeast extract
- 10 g NaCl
- 12 g Agar-Agar (for plates)
- adjust pH to 7.0
 "
"