Team:Bielefeld-Germany/Project/Outlook
From 2010.igem.org
(→Outlook) |
(→Outlook) |
||
| Line 1: | Line 1: | ||
{{Bielefeld_MainMenu_2010}} | {{Bielefeld_MainMenu_2010}} | ||
| + | {{Bielefeld_MainMenu_2010}} | ||
| + | <html> | ||
| + | <style type="text/css"> | ||
| + | .molFormulas { | ||
| + | width:200px; | ||
| + | text-align:center; | ||
| + | border:1px solid grey; | ||
| + | padding:10px; | ||
| + | background-color:#f9f9f9; | ||
| + | float:right; | ||
| + | margin-left:10px; | ||
| + | } | ||
| + | </style> | ||
| + | </html> | ||
= Outlook = | = Outlook = | ||
== The techniques’ potential == | == The techniques’ potential == | ||
| + | <html> | ||
| + | <div class="molFormulas"> | ||
| + | <h3>Capsaicin</h3> | ||
| + | <img src="http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=1548942" width="200"> | ||
| + | <img src="http://pubchem.ncbi.nlm.nih.gov/image/img3d.cgi?cid=1548942" width="200"> | ||
| + | <br/> | ||
| + | <a href="http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=1548942&loc=ec_rcs" target="_blank">see pubchem datasheet</a> | ||
| + | </div> | ||
| + | <div class="molFormulas"> | ||
| + | <h3>Acetosyringone</h3> | ||
| + | <img src="http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=17198" width="200"> | ||
| + | <img src="http://pubchem.ncbi.nlm.nih.gov/image/img3d.cgi?cid=17198" width="200"> | ||
| + | <br/> | ||
| + | <a href="http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=17198&loc=ec_rcs" target="_blank">see pubchem datasheet</a> | ||
| + | </div> | ||
| + | </html> | ||
| + | |||
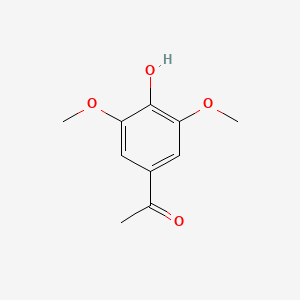
In our project we modulated the virA-gene from ’’A. tumefaciens’’, which is encoding a receptor for the plant-hormone acetosyringone, via error prone PCR. Slight mutations may alter the receptors conformation or its binding site. Both of these can cause shifts in specificity and sensitivity. This way we created a new receptor, which is capable of sensing capsaicin. The chemical formulas of capsaicin and acetosyringone are quite similar and there are other molecules of interest, that have some of the necessary properties, too. Thus, we suggest that it is possible to modulate VirA receptors in order to make them sense a variety of highly relevant compounds. | In our project we modulated the virA-gene from ’’A. tumefaciens’’, which is encoding a receptor for the plant-hormone acetosyringone, via error prone PCR. Slight mutations may alter the receptors conformation or its binding site. Both of these can cause shifts in specificity and sensitivity. This way we created a new receptor, which is capable of sensing capsaicin. The chemical formulas of capsaicin and acetosyringone are quite similar and there are other molecules of interest, that have some of the necessary properties, too. Thus, we suggest that it is possible to modulate VirA receptors in order to make them sense a variety of highly relevant compounds. | ||
| - | + | In other words, there are other candidates our sensing system may be applied to. All of them have similiar molecular structures as the vir-systems natural inducor acetosyringone. In the following we will present some of the most relevant compounds other than capsaicin, that may be upcoming targets of our research. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | === Dopamine === | + | <html><div style="clear:both;" /></html> |
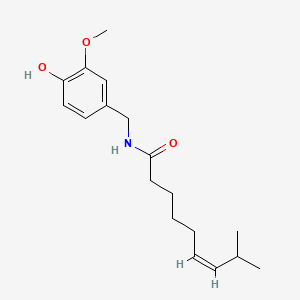
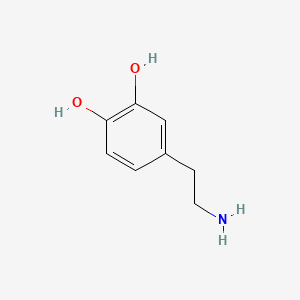
| - | + | == Dopamine == | |
| - | + | <html> | |
| + | <div class="molFormulas"> | ||
| + | <h3>Dopamine</h3> | ||
| + | <img src="http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=681" width="200"> | ||
| + | <img src="http://pubchem.ncbi.nlm.nih.gov/image/img3d.cgi?cid=681" width="200"> | ||
| + | <br/> | ||
| + | <a href="http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=681&loc=ec_rcs" target="_blank">see pubchem datasheet</a> | ||
| + | </div> | ||
| + | </html> | ||
Following some text ´bout it | Following some text ´bout it | ||
| - | === 3-Methoxytyramine === | + | <html><div style="clear:both;" /></html> |
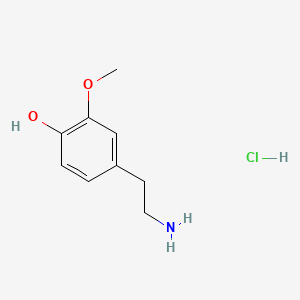
| - | + | == 3-Methoxytyramine == | |
| + | <html> | ||
| + | <div class="molFormulas"> | ||
| + | <h3>3-Methoxytyramine</h3> | ||
| + | <img src="http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=11957621" width="200"> | ||
| + | <img src="http://pubchem.ncbi.nlm.nih.gov/image/img3d.cgi?cid=11957621" width="200"> | ||
| + | <br/> | ||
| + | <a href="http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=11957621&loc=ec_rcs" target="_blank">see pubchem datasheet</a> | ||
| + | </div> | ||
| + | </html> | ||
Following some text ´bout it | Following some text ´bout it | ||
| - | === Homovanillic acid === | + | <html><div style="clear:both;" /></html> |
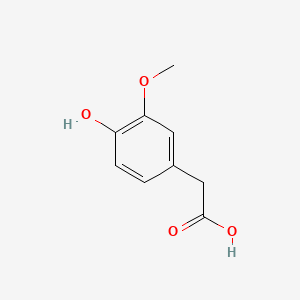
| - | + | == Homovanillic acid == | |
| - | + | <html> | |
| + | <div class="molFormulas"> | ||
| + | <h3>Homovanillic acid</h3> | ||
| + | <img src="http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=1738" width="200"> | ||
| + | <img src="http://pubchem.ncbi.nlm.nih.gov/image/img3d.cgi?cid=1738" width="200"> | ||
| + | <br/> | ||
| + | <a href="http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=1738&loc=ec_rcs" target="_blank">see pubchem datasheet</a> | ||
| + | </div> | ||
| + | </html> | ||
Following some text ´bout it | Following some text ´bout it | ||
| - | === Vanilmandelic Acid=== | + | <html><div style="clear:both;" /></html> |
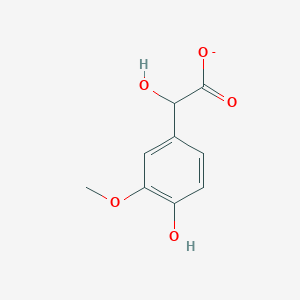
| - | + | == Vanilmandelic Acid == | |
| - | + | <html> | |
| + | <div class="molFormulas"> | ||
| + | <h3>Vanilmandelic Acid</h3> | ||
| + | <img src="http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=23615482" width="200"> | ||
| + | <img src="http://pubchem.ncbi.nlm.nih.gov/image/img3d.cgi?cid=23615482" width="200"> | ||
| + | <br/> | ||
| + | <a href="http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=23615482&loc=ec_rcs" target="_blank">see pubchem datasheet</a> | ||
| + | </div> | ||
| + | </html> | ||
Following some text ´bout it | Following some text ´bout it | ||
Revision as of 13:19, 23 October 2010
Contents |
Outlook
The techniques’ potential
In our project we modulated the virA-gene from ’’A. tumefaciens’’, which is encoding a receptor for the plant-hormone acetosyringone, via error prone PCR. Slight mutations may alter the receptors conformation or its binding site. Both of these can cause shifts in specificity and sensitivity. This way we created a new receptor, which is capable of sensing capsaicin. The chemical formulas of capsaicin and acetosyringone are quite similar and there are other molecules of interest, that have some of the necessary properties, too. Thus, we suggest that it is possible to modulate VirA receptors in order to make them sense a variety of highly relevant compounds.
In other words, there are other candidates our sensing system may be applied to. All of them have similiar molecular structures as the vir-systems natural inducor acetosyringone. In the following we will present some of the most relevant compounds other than capsaicin, that may be upcoming targets of our research.
Dopamine
Following some text ´bout it
3-Methoxytyramine
Following some text ´bout it
Homovanillic acid
Following some text ´bout it
Vanilmandelic Acid
Following some text ´bout it
 "
"