Team:LMU-Munich/Notebook/Apoptosis
From 2010.igem.org
(→9-16-2010) |
(→9-17-2010) |
||
| Line 3,197: | Line 3,197: | ||
== 9-17-2010 == | == 9-17-2010 == | ||
| - | + | <font color="#009933">Analysis of the transformation from yesterday</font> | |
| + | |||
| + | {| | ||
| + | |- | ||
| + | | | ||
| + | |1 | ||
| + | |3 | ||
| + | |5 | ||
| + | |6 | ||
| + | |9 | ||
| + | |10 | ||
| + | |- | ||
| + | |pellet | ||
| + | |no | ||
| + | |yes | ||
| + | |yes | ||
| + | |yes | ||
| + | |yes | ||
| + | |yes | ||
| + | |yes | ||
| + | |- | ||
| + | |100µl | ||
| + | |no | ||
| + | |yes | ||
| + | |yes | ||
| + | |yes | ||
| + | |yes | ||
| + | |yes | ||
| + | |yes | ||
| + | |} | ||
| + | |||
| + | <font color="#009933">again: transformation of ligation 1</font> | ||
| + | |||
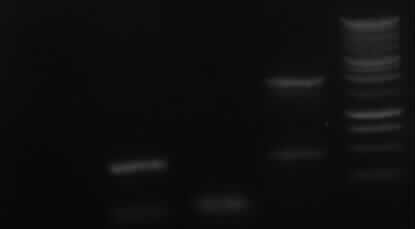
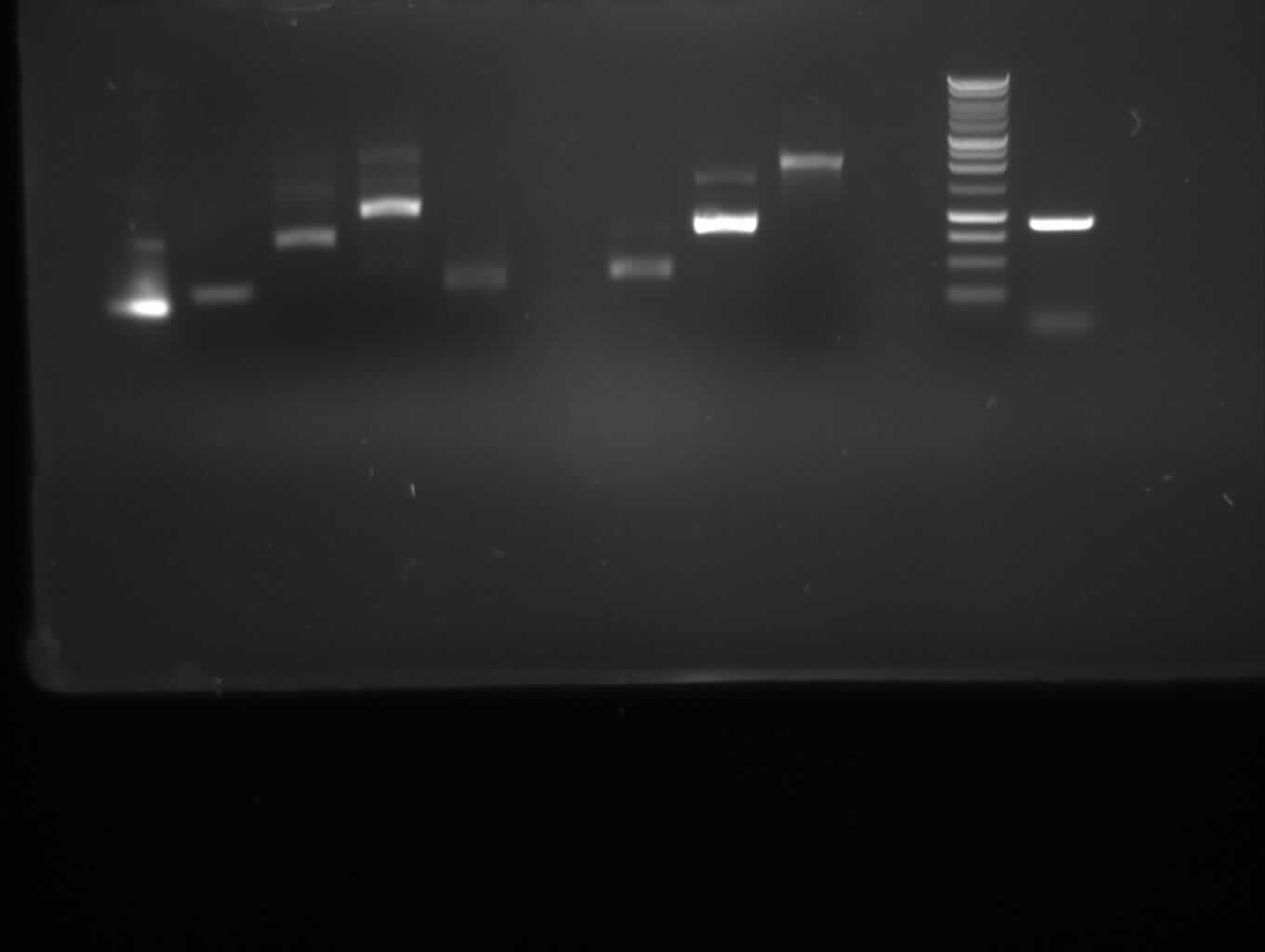
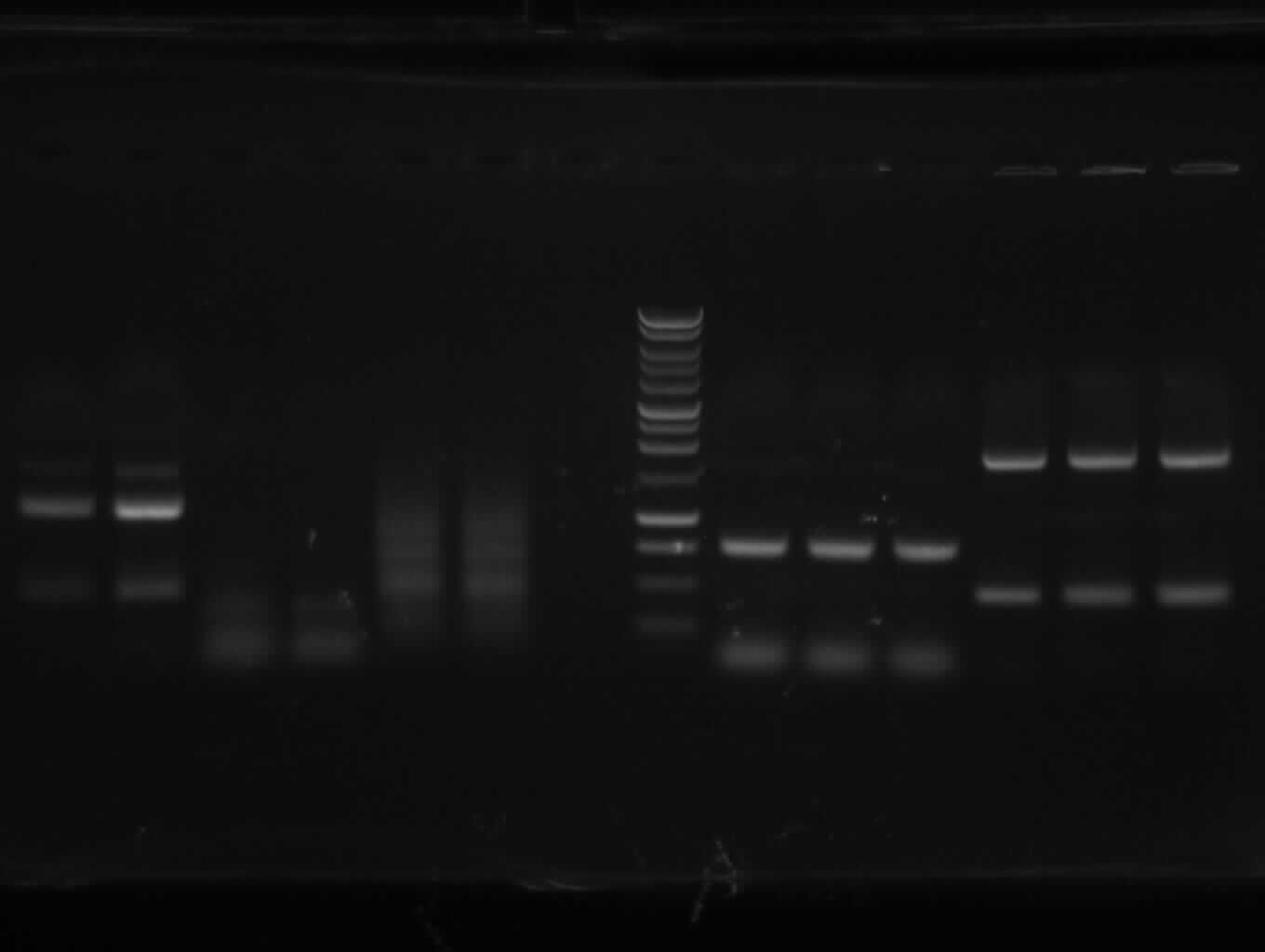
| + | <font color="#009933">Agarose gelelectrophoresis of yesterday's PCR 1,3,5,6,9,10(without mutation)</font> | ||
| + | |||
| + | -> right band for PCR 3,5,6,9,10; no band for PCR 1 | ||
| + | |||
| + | <font color="#009933">new PCR 1,5,6</font> | ||
| + | |||
| + | {| | ||
| + | |- | ||
| + | | | ||
| + | |1 | ||
| + | |5 | ||
| + | |6 | ||
| + | |- | ||
| + | |template | ||
| + | |pTRE Rev 0,5µl | ||
| + | |4a 1,5µl + 4b 1,5µl | ||
| + | |pcDNA3 0,5µl | ||
| + | |- | ||
| + | |primer | ||
| + | |1&2 2*2,5µl | ||
| + | |7&10 2*2,5µl | ||
| + | |11&12 2*2,5µl | ||
| + | |- | ||
| + | |buffer 10x | ||
| + | |5µl | ||
| + | |5µl | ||
| + | |5µl | ||
| + | |- | ||
| + | |dNTPs | ||
| + | |1µl | ||
| + | |1µl | ||
| + | |1µl | ||
| + | |- | ||
| + | |Pfu | ||
| + | |0,5µl | ||
| + | |0,5µl | ||
| + | |0,5µl | ||
| + | |- | ||
| + | |H<sub>2</sub>O | ||
| + | |26,75 µl | ||
| + | |24,25 µl | ||
| + | |26,75 µl | ||
| + | |- | ||
| + | |T<sub<m</sub> | ||
| + | |51°C | ||
| + | |50°C | ||
| + | |55°C | ||
| + | |} | ||
| + | |||
| + | pcr-program: standart Pfu [[Team:LMU-Munich/Notebook/Protocols/10 PCR with Pfu| 10 PCR with Pfu]] | ||
| + | |||
== 9-18-2010 == | == 9-18-2010 == | ||
weekend | weekend | ||
Revision as of 10:31, 17 September 2010
Some test text in bold
We created following tests:
- test4 Example of a table
this is also a table:
table with 3 cells
text
text
test text Knallroter Text test grüner text
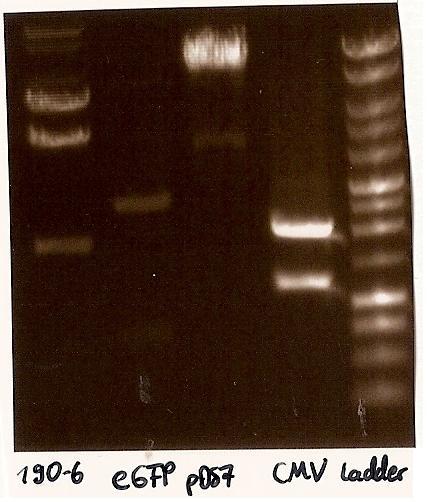
Transforming competent cells
- eGFP Biobrick: BBa_I714891 SDY_eGFP (Kanamycin)
- TEV recogn N Degron SF3 = pDS7 (Ampicillin)
- TEV p14 recogn = 190-6 (Ampicillin)
-> Protocol: (3 Transformation)
- We added 2 µl DNA
- We plated out 200 µl
- CMV-Promoter Biobrick: BBa_J52034
-> Protocol:(4 Plasmid extraction from cells)
- Prepared overnight culture, measured concentration of DNA
-> Poor results -> thrown away
New Plasmid Extraction
- CMV-Promoter Biobrick: BBa_J52034
-> Protocol: (4 Plasmid extraction from cells)
- Plasmid concentration: 143ng/µl
- 3 ml LB-Media + 4 µl Kanamycin
- Inoculated iangeimpft) with 1 colony of BBa_I714891 -> 37°C
- for 190-6 and pDS7: 10µl Ampicillin + 10 ml LB-Media + colony of plate
- for eGFP: 13,3 µl Kanamycin + 10 ml LB-Media + 1 colony of plate
-> mixed
- plus: EcoRI (10µg/µl): 0,5 µl resp. PstI (10µg/µl): 0,5 µl
- incubated at room temperature from 12:10 to 15:00, 1 hour at 37°C, 2 hours at 60°C
- frozen at -20°C
- 1 ml of "old" culture + 3 ml LB-Media + 4 µl Kanamycin -> 37°C
Plasmid Extraction of pDS7, eGFP, 190-6
-> Protocol: (4 Plasmid extraktion from cells)
- pDS7 (458ng/µl), eGFP (55ng/µl), 190-6 (193ng/µl)
Restriction digest of pDS7, eGFP, 190-6
- with EcoRI and PstI in buffer H (for testing DNA is correct)
-> Protocol: (5 Restriction digest)
- 10µg DNA: pDS7 (2µl), eGFP (15µl), 190-6 (10µl)
Plate colonies for plasmid extraction
- CMV (Kanamycin), eGFP (Kanamycin), pDS7 (Ampicillin), 190-6 (Ampicillin))
- PhiC31o plated on Ampicillin-Agar, stored at 37°C
50% Glycerol made
- for PhiC31o glycerol stock (produced later)
Inoculate CMV into LB medium with ampicillin
- CMV (BBa_J52034) from 10.8.2010 inoculated into LB medium with ampicillin, as falsly inoculated in Kanamycin
Agarosegelelectrophoresis with digestions
->Protocol (11 Agarose gel electrophoresis)
- Agarosegelelectrophoresis with the digestions (CMV, eGFP, pDS7, 190-6), 125V for 30 minutes and then for 20 minutes;
- expected DNA bands: 190-6 (4840bp, 1903bp), pDS7 (8027bp, 6bp), CMV (654 bp (Insert), 2079bp (Plasmid)), eGFP (720bp (Insert), 2750bp (Plasmid))
- Correct DNA bands for 190-6 (~4800bp, ~1900bp, ~6700bp (undigested plasmid)) and eGFP (~2000bp (Plasmid), ~750 bp (Insert)); CMV probably not digested (two bands; one probably normal, one supercoiled) and pDS7 not clear
Restriction digest from CMV and pDS7
-> Protocol (5 Restriction digest)
- Restriction digest from CMV (EcoR1, Pst1; 6µl DNA, buffer H) and pDS7 (EcoR1, Spe1; 2µl DNA, buffer B)
Agarosegelectrophoresis with digestions
->Protocol (11 Agarose gel electrophoresis)
- Agarosegelelectorphoresis for 30 minutes, 150V
- Expected DNA bands: CMV see above, pDS7 (3647bp, 3369bp, 1011bp, 6bp)
- false DNA bands CMV (~1200 bp, ~2000 bp) and pDS7 (~8000bp two bands, ~1100 bp); required to isolate a new colony for these two Plasmidextractions
Plated CMV on Ampicllin-Agar
- Plated the colony from CMV (BBa_J52034) for Plasmidextraction (Ampicillin), as falsly plated on Kanamycin
weekend
weekend
Planting colonies
- transfer 1 ml PhiC31o culture to new LB medium + Amp, 37°C
- pick up CMV and pDS7 colonies from plates and transfer to LB medium+Amp, 37°C
Plasmid Extraction of PhiC31o
->Protocol (4 Plasmid extraktion from cells)
- plasmid extraction of PhiC310
->27,5ng/µl DNA and second plasmid extraction of PhiC310 (i. o. to get more DNA); first eluation-step with first eluation-extraction
-> 60ng/µl DNA
Restriction digest
->Protocol (5 Restriction digest)
- restriction digest of PhiC310 with EcoR1 and Spe1
restriction digest in the thermo cycler (program "Verdau", see protocol)
Handling primers after arrival (1,2,3,4,5,6,11,12)
->Protocol (9 Handling primers)
PCR preparations
- 10mM dNTP mix made from 100 mM dATP, dGTP, dCTP, dTTP by taking 100µl of each and adding 600µl H 2 O
PCR 1 and 6
- PCR of the tet inducible CMV minimal promotor out of prevTRE (=PCR 1 with Primer 1 and 2) and SV40PA out of pcDNA3 (=PCR 6 with Primer 11 and 12)
->Protocol (10 PCR with Pfu)
Mixture:
Glycerolstock of PhiC31o
- Glycerolstock of the colony of PhiC31o for the plasmidextraction
Plate CMV and pDS7 colonies on Ampicillin-Agar
- colonies for plasmidextraction of CMV and pDS7 plated on Ampicillinplates
Plasmid Extraction of CMV and pDS7
- plasmidextraction of CMV (2,5ng/µl) and pDS7 (10ng/µl) the A260/A280 value was 1.333, which means that it was 90% Protein and only 10% DNA (should be 1,8); new plasmidextraction needed
new overnight cultures of CMV and pDS7 for a new plasmidextraction made
Agarose gel electrophoresis
-> Protocol (11 Agarose gel electrophoresis)
- Agarose gel electrophoresis of the restriction digest of PhiC31o and PCR 1 and 6
- the right bands found for PhiC31o (~2900,~2400,~250)
- the right band found for PCR1 (~450)
- no band found for PCR6; new electrophoresis needed with more DNA loaded
- new agarose gel electrophoresis from PCR6 with 5µl DNA instead of 3µl (image not yet shown)
- the right band found for PCR6 (~200)
New overnight cultures of CMV and pDS7
- the overnight colonies didn't grow; new colonies (CMV and pDS7) picked from plate and inoculated in LB Ampicillin
PCR purification of PCR 1 and 6
-> Protocol (12 Gel extraction or PCR Clean up)
- DNA concentration of the PCR 1 and 6 products measured: PCR1: 410ng/µl (A260/A280=1.253) PCR6: 568ng/µl (A260/A280=1.275)
- PCR Purification with Promega Kit
-> PCR1: 230ng/µl (A260/A280=1.769)
-> PCR6: 37.5ng/µl (A260/A280=1.667)
Plasmid Extraction of CMV and pDS7
-> Protocol (4 Plasmid extraction from cells)
- Plasmid extraction of CMV (97.5ng/µl; A260/A280=1.857) and pDS7 (212ng/µl; A260/A280=1.848)
Restriction digestion
-> Protocol (5 Restriction digestion)
- Restriction digestion of CMV (EcoR1 + Pst1; 10µl DNA, buffer H) and pDS7 (EcoR1 + Spe1; 5µl DNA, buffer B)
-> expected DNA bands: CMV: 2079bp (plasmid) + 654bp (Insert); pDS7: 7022bp + 1011bp
Agarose Gel electrophoresis of digested CMV and pDS7
-> Protocol (11 Agarorse gel electrophoresis)
-> right DNA bands for pDS7 (~7000bp, ~1000bp)
-> false DNA bands for CMV
- Starting PCR 2a and 2b (replication and mutagenesis of pDS7): 3 µl DNA and 50°C Annealing Temperatur (other same as 8-16-2010)
Agarose gel electrophoresis of PCR 2a and 2b
-> Protocol (11 Agarose gel electrophoresis)
(150V, 30min)
-> the right bands for PCR2a (~300bp) and PCR2b (~700bp)
- New agarose gel electrophoresis with all of the PCR product for gel extraction (150V, 30min)
Gel extraction of the DNA from PCR2a and PCR2b
-> Protocol (12 Gel extraction or PCR Clean up)
- DNA concentration measured; problem with nanodrop as too low concentration; lyophille used to reduce volume
- DNA concentration measured again: PCR2a: 70ng/µl A260/A280=1.647; PCR2b: 45ng/µl A260/A280=1.5
PCR 3 (joining PCR of 2a and 2b)
- PCR3 (the joining PCR of PCR2a and 2b; Joining of the TEVrecogn-N-Degron-SF3 part) done: 1.3 µl of PCR2a and 4.7 µl of PCR2b makes 300ng of a 1:1 solution of both to be joined DNA parts. Annealing temperature: 50°C
-> Protocol (10 PCR with Pfu)
Agarose gel electrophoresis of PCR3
left column: marker; most right column: PCR3
-> Protocol: 11 Agarose gel electrophoresis (150V, 30min)
-> expected band: ~1000bp
-> false band: ~500bp
- probable reason: mini photometre was influenced by gel extraction chemicals, therefore it measured false DNA concentrations and false template masses were calculated
-> New 2a and 2b PCR
New PCR (2a and 2b)
-> Protocol: 10 PCR with Pfu
(see 8-18-2010, but 35,5µl water)
weekend
weekend
Agarose gel electrophoresis of PCR 2b
-> Protocol: 11 Agarose gel electrophoresis
- expected band: 700bp
-> no band shown on gel -> new PCR 2b
PCR 2b
- start PCR 2b with PCR 2b from 8-13-10 as template ( 1:20 and 1:100 diluted; 1µl)
-> Protocol: 10 PCR with Pfu
- annealing temperature: 50°C; amount of water: 37,5µl
Agarose gel electrophoresis of PCR 2b 1:20 and 1:100
-> Protocol: 11 Agarose gel electrophoresis
- expected bands: each ~ 700bp
- false bands: ~ 200bp
-> new PCR with 2ng, 5ng, 10ng template pDS7
- pDS7 1:100 diluted(-> 2,1 ng/µl)
Mixture:
-> Protocol: 10 PCR with Pfu
PCR 2a gel extraction
- Quaigen kit (QuaiexII)
-> Protocol: 14 QIAEX II gel extraction
Start 3 CMV overnight cultures
agarose gel electrophoresis of PCR 2b
-> Protocol: 11 Agarose gel electrophoresis
- expected bands: right bands with 2ng and 5ng template (~700bp), no band with 10ng template
CMV plasmid extraction
-> Protocol: 4 Plasmid extraction from cells
Plasmid extractionof 3 different overnight cultures.
- results:
CMV restriction digestion
-> Protocol: 5 Restriction digestion
- CMV restriction digest: EcoRI, PstI, buffer H
PCR 2b gel extraction
- PCR2b was gel extracted (with Qiagen gel extraction kit), 17.5 ng/µl a260/A280= 1.750
-> Protocol: 14 QIAEX II gel extraction
PCR 3 (fusion of 2a and 2b)
- PCR3: conducted again at 52°C annealing temperature
-> Protocol: 10 PCR with Pfu
agarose gel electrophoresis of CMV digestion
- agarose gel electrophoresis (150V, 25 min) of the CMV digestion
-> bands are wrong again ( ~ 1200bp, 2000bp)
Agarose gel electrophorese of PCR 3
-> Protocol: 11 Agarose gel electrophoresis
- expected band: ~1000bp
- false band: ~400bp
Plasmid extraction of ccdB tet and ccdB strep
Plasmid extraction of pSB1C3 with BBa_P1010
-> Protocol: 4 Plasmid extraction from cells
- results:
- plate ccdB with ampicilline, chloramphenicol, tetracycline resistance on LB agar with appropiate antibiotic.
- Overnight culture of ccdB with kanamycine resistance in LB medium with kanamycine
PCR 7a, 7b, 9, 10
->Protocol: 10 PCR with Pfu
Standard PCR; annealing temperature: 60°C
Agarose gelelectrophoresis of PCR 7a, 7b, 9, 10
->Protocol: 11 Agarose gel electrophoresis
- 150V, 25min
Plasmid extraction of ccdB kan
-> Protocol: 4 Plasmid extraction from cells
-result: concentration: 25ng/µl; A260/A280= 2,0
New PCR 7a, 7b, 9, 10
Mixture
Program:
gradient PCR, 42-69°C annealing temp.
->Protocol: 10 PCR with Pfu
Overnight culture of ccdB amp, tet, cam
Inoculate one colony each in 5ml medium with approptraite antibiotic.
Agarose gel electrophoresis of PCR 7a, 7b, 9, 10
->Protocol: 11 Agarose gel electrophoresis
150V, 25min, 75mA
from left to right: 7a, 7b, 9, 10, Marker
Plasmid extraktion of ccdB amp, tet, cam
->Protocol: 4 Plasmid extraction from cells
results:
Mixture:
- 2ng template: see 26-8-10
- 4ng template: see 26-8-10, but 2µl template and 35,25µl water
-> Protocol: 10 PCR with Pfu
-> Protocol: 5 Restriction digestion
- only 90min 37°C incubation
- EcoRI, PstI, Buffer H
Agarose gelelectrophoresis of PCR 7a, 9, ccdB restriction digestion
150v, 25min, 75mA
-> Protocol: 11 Agarose gel electrophoresis
results:
- PCR7a, 9: false band at 200bp
- ccdB: each digestion leads to a right band with ~ 650bp
weekend
weekend
New PCR 7a and 9
PCR 7: Annealing Temperature 60°C - 25 x 1 min Annealing time and 5x 1,30 min Annealing time
PCR 9: Annealing Temperature 55°C - 25 x 1 min Annealing time and 5x 1,30 min Annealing time
->Protocol: 14 QIAEX II gel extraction
results:
-> Protocol: 11 Agarose gel electrophoresis
150V, 25min
- results:
- 7a: no band shown
- 9: false band (~200bp)
New PCR 3
-> Protocol: 10 PCR with Pfu
- new method: standard PCR without primers (10 cycles, 56°C annealing temp.)
- then add 2,5µl of primer 3 and 6
- 30 cycles standard PCR (54°C annealing temp.)
Agarose gel electrophoresis of PCR 4a, PCR4b, PCR3(Pfu), PCR3(Phusion)
-> Protocol: 11 Agarose gel electrophoresis
150V, 25min
- results:
- PCR4a(2.5ng template), PCR4a(5ng template),PCR4b(2.5ng template), PCR4b(5ng template), PCR3(Pfu): no band shown
- PCR3 (Phusion): right band (~1000bp)
New PCR PCR4a, PCR4b, PCR7a, PCR9
-> Protocol: 10 PCR with Pfu
PCR mixture for PCR4a, PCR4b
Standard PCR program with annealing temperature PCR4a: 51.1°C, PCR4b: 48.5°C.
-> Protocol: 15 PCR with Phusion
PCR mixture for PCR7a, PCR9
PCR program: Phu62
Agarose gel electrophoresis of PCR 3, PCR7a, PCR9 for gel extraction
-> Protocol: 11 Agarose gel electrophoresis
120V, 30min
- results:
- PCR3; right band (~1000bp) and side-product
- PCR7a: no band
- PCR9: right band (~800bp) and side-product
Gel extraction of the DNA from PCR3 and PCR9
-> Protocol (12 Gel extraction or PCR Clean up)
results:
PCR 9: 22,5ng/µl; A260/A280=1,8
PCR 3: 22,5ng/µl; A260/A280=2,25
New PCR 4a, 4b, 7a with DreamTaq
-> Protocol: 16 PCR with DreamTaq
PCR mixture for PCR7a
Primers for PCR 7a: 13,14
Annealing temp: 60°C
PCR mixture for PCR4a,4b
PCR 4a
Primers for PCR 4a: 7,8
Primers for PCR 4b: 9,10
Annealing temp: 50°C
PCR program:
Agarose gel electrophoresis of PCR4a, PCR4b, PCR7 (DreamTaq)
-> Protocol: 11 Agarose gel electrophoresis
150V, 25min
results: no product
New PCR 4a, 4b with DreamTaq, Pfu, with concentration gradient and touch-down PCR
-> Protocol: 16 PCR with DreamTaq; 10 PCR with Pfu
PCR mixture for DreamTaq
PCR mixture for Pfu
Primers for PCR 4a: 7,8; PCR 4b: 9,10
-> Protocol: Thermal cycler program: Touch down
Agarose gel electrophoresis of PCR4a, PCR4b
-> Protocol: 11 Agarose gel electrophoresis
150V, 25min
from left to right: 4a: P1, P2, P3, D1, D2, D3; ab: P1, P2, P3, D1, D2, D3
key:
"P"= PCR with Pfu
"D"= PCR with DreamTaq
"1"= low template concentration
"2"= middle template concentration
"3"= high template concentration
expected bands:
Agarose gel electrophorese of PCR 4a P2, 4b P2
-> Protocol: 11 Agarose gel electrophoresis
- 120V, 45min, 1,5% Agarose gel
Agarose gel electrophoresis of (from left to right) PCR4aP2, Marker and PCR4bP2
PCR Agarose gel extraction
-> Protocol: 14 QIAEX II gel extraction
results:
template: 190-6, Primer 13,14
Mixture with Pfu
PCR mixture with Phusion
-> Protocol: 15 PCR with Phusion
New PCR 4a, 4b with Pfu
-> Protocol: 10 PCR with Pfu
-> Protocol: Thermal cycler program: Touch down
Mixture see 9-1-10, twice 4a and 4b
Agarose gel electrophorese of PCR 4a, 4b, 7a, gel extracted 4a and 4b
-> Protocol: 11 Agarose gel electrophoresis
- 25min, 150V
from left to right: 4a*, 4a, 4b*, 4b, 7a Phusion, 7a Pfu, Ladder
-> result: 4b, 4b*: right bands (~330bp)
-remain: false bands/no band
from left to right: ladder, 4 columns pathway, 4a gelextr., 4b gelextr.
-> results: slight right bands for 4a and 4b, no "primer clouds" anymore.
Overlapping PCR 5 with Pfu and Phusion
template: 4a, 4b Primer 7,10
Mixture with Pfu
-> Protocol: 10 PCR with Pfu
PCR program: standard PCR program for Pfu, Annealing temperature: 54°C
PCR mixture with Phusion
-> Protocol: 15 PCR with Phusion
PCR program: standard PCR program for Phusion, Annealing temperature: 58°C
New PCR7a with Pfu
template: 190-6; Primer: 13,14
Mixture with Pfu
-> Protocol: 10 PCR with Pfu
PCR program: standard PCR program for Pfu, Gradient: 54.4°C, 57.8°C, 61.4°C, 65.0°C
-> results:
-7a: only "primer cluods"
-5 Pfu: no defined product (slurred?)
-5 Phusion: "primer clouds"
weekend
weekend
charges for sequencing
every charge 7µl
template: 4a, 4b; 15ng
Primer 7,10
Mixture
-> Protocol: 10 PCR with Pfu
PCR program: standard PCR program for Pfu, Annealing temperature: 46.1°C,48.5°C, 51.1°C
-> results: "primer clouds"
-> results: "primer clouds"
every charge 7µl
Agarose gel electrophorese of PCR gel extractions
150V, 25min
-> Protocol: 11 Agarose gel electrophoresis
-result:
from left to right: PCR 1,2a,2b,3,4a,/,7b,9,10,/,ladder,pathway
new PCR for PCR6
PCR mixture for PCR6
-> Protocol: Thermal cycler program: Touch down, 62°C-52°C, 30 cycles by 55°C
Restriction digestion of eGFP, PCR6, ccdBamp
as prepatation for ligation
-> Protocol 5 Restriction digestion
1:30h 37°C, 20min 80°C
Agarose gel electrophoresis of PCR6
-> Protocol: 11 Agarose gel electrophoresis
25min, 150V
Concentration of DNA in Ligation 1:
-> Protocol 18 competent cells2
The incubation time for the cells is here 1 hour.
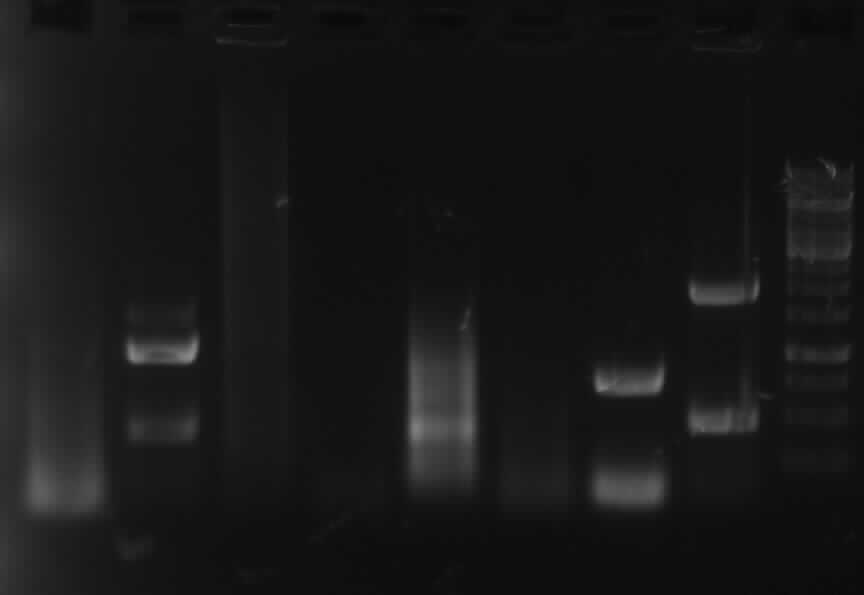
Agarose gel electrophoresis of PCR 1, 3, 4a, 5, 7a, 9
Agarose gel electrophoresis of PCR 1, 3, 4a, 5, 7a, 9
-> protocol 11 Agarose gel electrophoresis
results: "primer-clouds", PCR 9: no band
-> Protocol 4 Plasmid extraction from cells
Eluated with H2O instead of the Eluation Buffer.
New PCR 1, 3, 4a, 5, 7a, 9, 10 with phusion
+ in each assay:
dNTP mix: 1µl, 5x Phusion buffer: 10µl, DMSO:1.5µl, Phusion:0.5µl
sum: 50µl
Program: standard Phusion PCR, 29 cycles; annealing temperatures: see above
-> Protocol: 15 PCR with Phusion
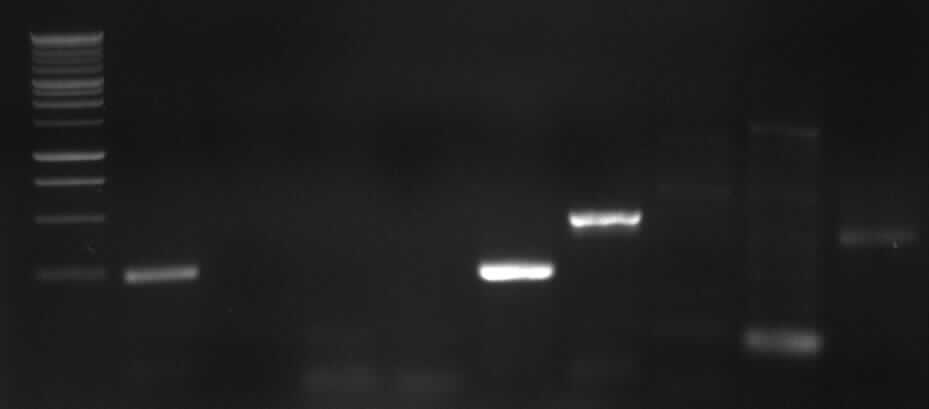
[from left to right: PCR 1, 3, 4a, 5, 7a, 9, 10, Ladder]
PCR3, 9, 10 with right bands.
New PCR 1, 4a, 4b, 5, 7a with phusion
+ each assay with dNTP-mix (1µl), 5xBuffer (10µl, DMSO (1.5µl), Phusion (0.5µl)
-> sum: 50µl
-> Protocol Touch down 59 with phusion, 30 cycles with gradient appropriate for the annealing temperatures above.
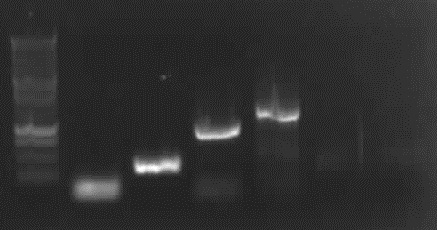
Agarose gel electrophoresis of PCR 1, 4a, 4b, 5, 7a
[From left to right: Ladder, 1, 4a, 4b, 5, 7a]
Only 4a has been amplified successfully.
from left to right: PCR 3, band ~700bp, PCR 4a, band ~300bp, PCR 5, bands ~550bp (5*), ~650bp (5), PCR 9, band ~800bp, PCR 10, band ~1900bp
results:
-> Protocol 12 Gel extraction or PCR Clean up (Promega kit)
weekend
weekend
charges for sequencing (retry of 6.9.)
every charge 7µl
template: p190-6
Primer 13,14
Mixture
PCR program: touchdown PCR with Taq
with 2ng and 4 ng template
total: 100µl each, divided into 5 charges
program:
Agarose gelelectrophoresis of PCR 7a
bands 6 to 10: 4ng of template DNA
-> no bands
new PCR 7a and PCR 8 (without mutation)
with mastermix, without DMSO
7a diluated and undiluated: divided into 3 charges
7a diluated: 1,2,3; 7a undiluated: 4,5,6
program:
Plasmid extraction of ccdB (amp) and ccdB (cam)
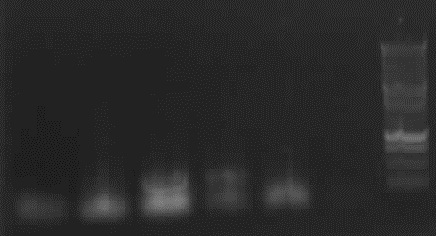
Agarose gelelectrophoresis of PCR 7a diluted &7a undiluted & "8"(without mutation)
weak right band (with fuzz)for 7a undiluated 56°C
-> new PCR 7a and "8"
with mastermix Taq
template: 190-6, 1:10 and 1:5
temp: 56°C and 58°C
2 charges each: one for 56°C and one for 58°C annealing temperature
EcoR1 + Pst1 with Buffer H; 50ng DNA
Agarose gelelectrophoresis of PCR 7a 1-4 & "8" 1-4(without mutation)
-> bad results
purification and restriction digestion
Ligation with pSB1C3 (2072bp)
plus 1 µl T4 ligase in each charge
with mastermix Taq 56°C
sum: 20µl each
pcr-program:
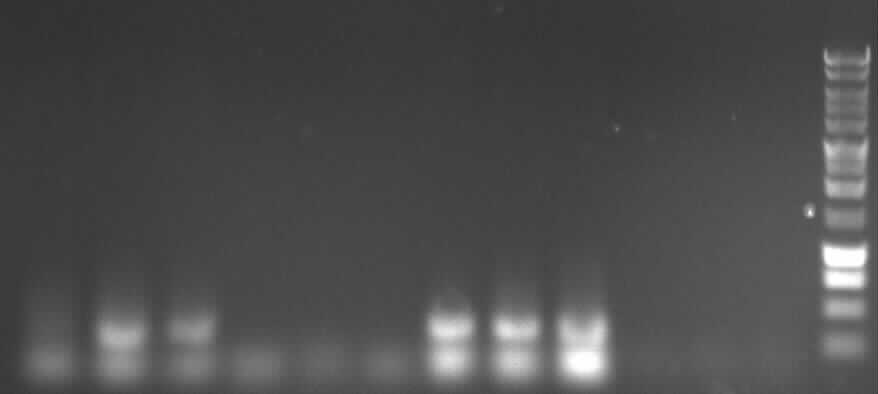
Agarose gelelectrophoresis of PCR 7a-1, PCR 7a-2, PCR 8-1, 8-2 and of all three PCR 6 we ever made (in order to control whether we have extracted the right fragment (237) or just the primerdimers)
transformation of the ligations 1,3,51, 52, 6, 9, 10
->plated on plates with chloramphenicol
again PCR 1,3,5,6,9,10 in order to increase the profit (Ausbeute) for ligation
pcr-program:
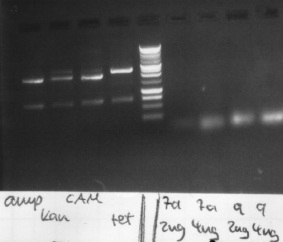
Analysis of the transformation from yesterday
again: transformation of ligation 1
Agarose gelelectrophoresis of yesterday's PCR 1,3,5,6,9,10(without mutation)
-> right band for PCR 3,5,6,9,10; no band for PCR 1
new PCR 1,5,6
pcr-program: standart Pfu 10 PCR with Pfu
weekend
weekend
text
text
text
text
text
weekend
weekend
text
text
text
text
text
weekend
weekend


![]()
![]()







![]()
![]()

![]()
Apoptosis Notebook
Contents
Week Days
Monday
Tuesday
Wednesday
Thursday
Friday
Saturday
Sunday
31
8-02-2010
8-03-2010
8-04-2010
8-05-2010
8-06-2010
8-07-2010
8-08-2010
32
8-09-2010
8-10-2010
8-11-2010
8-12-2010
8-13-2010
8-14-2010
8-15-2010
33
8-16-2010
8-17-2010
8-18-2010
8-19-2010
8-20-2010
8-21-2010
8-22-2010
34
8-23-2010
8-24-2010
8-25-2010
8-26-2010
8-27-2010
8-28-2010
8-29-2010
35
8-30-2010
8-31-2010
9-01-2010
9-02-2010
9-03-2010
9-04-2010
9-05-2010
36
9-06-2010
9-07-2010
9-08-2010
9-09-2010
9-10-2010
9-11-2010
9-12-2010
37
9-13-2010
9-14-2010
9-15-2010
9-16-2010
9-17-2010
9-18-2010
9-19-2010
38
9-20-2010
9-21-2010
9-22-2010
9-23-2010
9-24-2010
9-25-2010
9-26-2010
39
9-27-2010
9-28-2010
9-29-2010
9-30-2010
10-01-2010
10-02-2010
10-03-2010
8-02-2010
8-03-2010
- test5
8-04-2010
header 1
header 2
header 3
row 1, cell 1
row 1, cell 2
row 1, cell 3
row 2, cell 1
row 2, cell 2
row 2, cell 3
8-05-2010
H2Oddes
10,3 µl
RE10 + Buffer H
2,0 µl
acetylated BSA
0,2 µl
DNA
6,0 µl
apple banana peaches
green yellow red
8-06-2010
8-07-2010
8-08-2010
test
8-09-2010
farbnummern für farbige schrift: http://html.nicole-wellinger.ch/hilfen/farbenverzeichnis.html
8-10-2010
Plasmid Isolation
8-11-2010
Prepared overnight culture of eGFP BBa_I714891
Prepared overnight culture of 190-6 and pDS7 and eGFP (BBa_I714891) in falcons
Restriction digestion of CMV-Promoter BBa_J52034 with EcoRI and PstI
H2Oddest, sterile
10,3 µl
RE10 + Buffer H
2,0 µl
acetylated BSA (18ng/µl)
0,2 µl
DNA (0,143µg/µl)
6,0 µl
Prepare new/fresh overnight culture of CMV-Promoter Biobrick: BBa_J52034
8-12-2010
8-13-2010
8-14-2010
8-15-2010
8-16-2010
H2Oddest, sterile
0 µl
Buffer B
2,0 µl
BSA (1:10)
2 µl
DNA (0,06µg/µl)
15,0 µl
EcoR1
0,5 µl
Spe1
0,5µl
pTRERev (0,15µg/µl)
pcDNA3 (0,6 µg/µl)
Primer
2*2,5µl (P1+P2)
" (P11+P12)
300ng template
0,5µl
2µl
10x Buffer Pfu
5µl
"
dNTP Mix
1µl
"
Pfu Polymerase (3u/µl)
0,5µl
"
H2O
40,5µl
39µl
summ
52,5µl
52,5µl
Programme:
Denaturation
95°C
2min
30 times:
Denaturation
95°C
1min
Annealing
45°C
30sec
Extension
73°C
2min
Final Extension
73°C
5min
Soak (end)
12°C
infinite
bacterial culture
800µl
Glycerol (50%)
500µl
8-17-2010


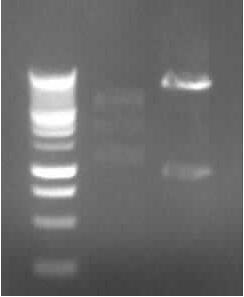
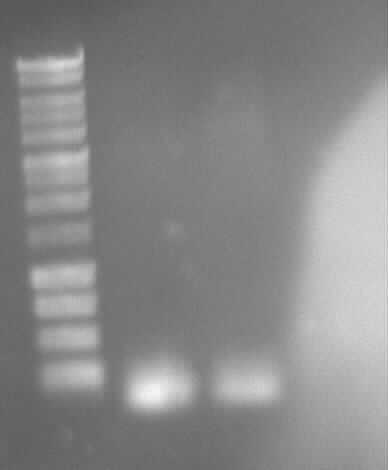
Agarose gel electrophoresis of (from left to right) PhiC31o, PCR1 and PCR6
Agarose gel electrophoresis of (from left to right) PhiC31o, PCR1 and PCR6 which shows that PCR1 is between 250 and 500 bp
8-18-2010
8-19-2010
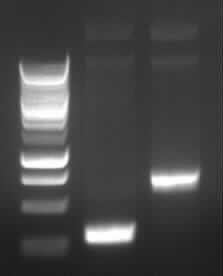
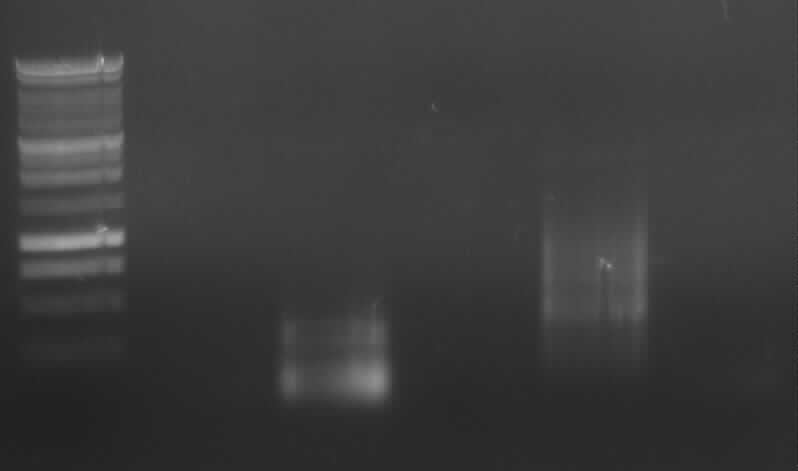
Agarose gel electrophoresis of (from left to right) PCR2a and PCR2b
8-20-2010
8-21-2010
8-22-2010
8-23-2010
PCR 2b with 2ng, 5ng, 10ng template pDS7
2ng
5ng
10ng
Primer
2*2,5µl (P5+P6)
2*2,5µl (P5+P6)
2*2,5µl (P5+P6)
10x Buffer Pfu
5µl
5µl
5µl
dNTP Mix
1µl
1µl
1µl
template
pDS7 (dil.)
1µl
2,5µl
5µl
Pfu Polymerase (3u/µl)
0,5µl
0,5µl
0,5µl
DMSO
1,25µl
1,25µl
1,25µl
H2O
33,25µl
30,25µl
25,25µl
sum
8-24-2010
PCR2a
0.9 µl
PCR2b
0.6 µl
primer3
2.5 µl
primer6
2.5 µl
dNTPs
1 µl
Pfu
0.5 µl
10xbuffer
5 µl
H2O
37 µl
8-25-2010
ccdB tet:
50ng/µl;
A260/A280= 1,818
Plate ccdB amp, cam, tet
Overnight culture of ccdB kan
PCR nr.
template
concentration
dilution
primer
7a
190-6
~200ng/µl
1:100
13,14
7b
190-6
~200ng/µl
1:100
15,16
9
eGFP
55ng/µl
1:25
20,21
10
PhiC31o
20ng/µl
1:10
22,23
Mixture
template (~2ng)
1µl
Pfu
0,5µl
Primer *2
2,5µl *2
10x buffer
5µl
dNTP Mix
1µl
H2O
37,5µl
sum
50µl
8-26-2010
PCR nr.
expected bands
result
7a
850bp
no band
7b
402bp
false band (200bp)
9
808bp
no band
10
1888bp
no band
template (~2ng)
1µl
Pfu
0,5µl
Primer *2
2,5µl *2
10x buffer
5µl
dNTP Mix
1µl
DMSO
1,25µl
H2O
36,25µl
sum
50µl
8-27-2010
PCR nr.
expected bands
result
7a
850bp
no band
7b
402bp
right band (~400bp)+ false band (~150bp)
9
808bp
false band (~200bp)
10
1888bp
right band (~1900bp)+false band (~500bp)
Plasmid
concentration
A260/A280
ccdB amp
57,5 ng/µl
1,917
ccdB cam
70,0 ng/µl
1,867
ccdB tet
50,0 ng/µl
1,818
New PCR 7a, 9
Restriction digestion of ccdB amp, kan, cam, tet
template
volume
mass
ccdB amp
16µl
930ng
ccdB cam
14,3µl
1µg
ccdB tet
16µl
800ng
ccdB kan
16µl
400ng
8-28-2010
8-29-2010
8-30-2010
Mixture
template (~4ng)
2µl
Pfu
0,5µl
Primer *2
2,5µl *2
10x buffer
5µl
dNTP Mix
1µl
DMSO
1,25µl
H2O
35,25µl
sum
50µl
-> Protocol: 10 PCR with Pfu
PCR program
Gel extraction of PCR 7b, 10
PCR nr.
concentration
A260/A280
7b
10 ng/µl
2,0
10
17,5 ng/µl
1,4
Agarose gel electrophoresis of new PCR 7a, 9
PCR2a
0.9 µl
PCR2b
0.6 µl
dNTPs
1 µl
Pfu
0.5 µl
10xbuffer
5 µl
DMSO
1,25µl
H2O
36,75 µl
sum
45µl
8-31-2010
template
37.25 µl (200ng)
dNTPs
1 µl
Pfu
0.5 µl
10xbuffer
5 µl
DMSO
1,25µl
sum
50µl
template
4 µl (8ng)
dNTPs
1 µl
Phusion
0.5 µl
5xbuffer
10 µl
DMSO
1,25µl
H2O
28.25 µl
sum
50µl
98°C
1 min
98°C
10 sec
62°C
20 sec
73°C
30 sec
return to step 2 for 29 cycles
73°C
10 min
12°C
forever
template
5 µl (10ng)
dNTPs
5 µl
DreamTaq
0.33 µl
10xbuffer
5 µl
DMSO
1,25µl
H2O
28.5 µl
sum
50µl
template
36,5 µl (180ng HeLa cDNA)
dNTPs
5 µl
DreamTaq
0.33 µl
10xbuffer
5 µl
DMSO
1,25µl
sum
50µl
95°C
1 min
95°C
30 sec
50/60°C
30 sec
72°C
1 min (1kb/min)
return to step 2 for 29 cycles
72°C
10 min
12°C
forever
9-01-2010
Concentration
Low
Middle
High
template
5 µl (1:1000)
31.75µl (1:1000)
2µl (1:10)
dNTPs
5 µl
DreamTaq
0.33 µl
10xbuffer
5 µl
DMSO
1,25µl
H2O
26.75 µl
0
29.75
sum
50µl
Concentration
Low
Middle
High
template
5 µl (1:1000)
37.25µl (1:1000)
2µl (1:10)
dNTPs
1 µl
Pfu
0.5 µl
10xbuffer
5 µl
DMSO
1,25µl
H2O
32.25 µl
0
35.25 µl
sum
50µl
9-02-2010
- Cut out bands at ~~ 350bp and extract
PCR nr.
concentration
A260/A280
4aP2
12,5 ng/µl
1,67
4bP2
72,5 ng/µl
1,53
New PCR 7a with Pfu and Phusion
template (~2ng)
1µl
Pfu
0,5µl
Primer *2
2,5µl *2
10x buffer
5µl
dNTP Mix
1µl
DMSO
1,25µl
H2O
36,25µl
sum
50µl
-> Protocol: 10 PCR with Pfu
template (~2ng)
1,0 µl
dNTPs
1 µl
Phusion
0.5 µl
5xbuffer
10 µl
DMSO
1,25µl
H2O
31,25µl
sum
50µl
9-03-2010
PCR4a (~15ng)
1.2µl
PCR4b (~15ng)
2µl (1:10)
Pfu
0.5µl
Primer *2
2.5µl *2
10x buffer
5µl
dNTP Mix
1µl
DMSO
1,25µl
H2O
34.05µl
sum
50µl
PCR4a (~15ng)
1.2µl
PCR4b (~15ng)
2µl (1:10)
dNTPs
1 µl
Phusion
0.5 µl
5xbuffer
10 µl
DMSO
1,25µl
H2O
29.05µl
sum
50µl
template (~2ng)
1µl
Pfu
0.5µl
Primer *2
2.5µl *2
10x buffer
5µl
dNTP Mix
1µl
DMSO
1,25µl
H2O
36.25µl
sum
50µl
9-04-2010
9-05-2010
9-06-2010
name
4a-7
4a-8
4b-9
4b-10
3-3
3-6
6-11
6-12
DNA
4a; 2.4µl
4a; 2.4µl
4b; 0.5µl
4b; 0.5µl
3; 2µl
3; 2µl
6; 0.5µl
6; 0.5µl
primer [1pmol/µl]
7; 3.2µl
8; 3.2µl
9; 3.2µl
10; 3.2µl
3; 3.2µl
6; 3.2µl
11; 3.2µl
12; 3.2µl
Tris (10mM); pH 8.2
1.4µl
1.4µl
3.3µl
3.3µl
1.8µl
1.8µl
3.3µl
3.3µl
New overlapping PCR 5 with Pfu
PCR4a (~7ng)
0.56µl
PCR4b (~8ng)
1.1µl (1:10)
Pfu
0.5µl
Primer *2
2.5µl *2
10x buffer
5µl
dNTP Mix
1µl
DMSO
1,25µl
H2O
36.6µl
sum
50µl
New PCR 7a with Pfu
new dilution of 190-6; 1:100
template[2ng/µl]
1µl
2µl
pfu polymerase
0.5µl
0.5µl
primer13
2.5µl
2.5µl
primer14
2.5µl
2.5µl
buffer
5µl
5µl
dNTP Mix
1µl
1µl
DMSO
1,25µl
1.25µl
H2O
36.25µl
35.25
sum
50µl
50µl
Agarose gel electrophoresis of 7a
charges for sequencing for PCR 1,7b and 9
name
1-1
1-2
7b-15
7b-16
9-20
9-21
10-22
10-23
DNA
1[1:10]; 1.74µl
1[1:10]; 1.74µl
7b; 3µl
7b; 3µl
9; 1.78µl
9; 1.78µl
10; 3.8µl
10; 3.8µl
primer [1pmol/µl]
1; 3.2µl
2; 3.2µl
15; 3.2µl
16; 3.2µl
20; 3.2µl
21; 3.2µl
22; 3.2µl
23; 3.2µl
Tris (10mM); pH 8.2
2.06µl
2.06µl
0.8µl
0.8µl
2.02µl
2.02µl
0µl
0µl
9-07-2010
PCR nr.
1
2a
2b
3
4a
7b
9
10
expected band (bp)
492
332
772
1087
330
402
808
1888
shown band(s)
550,200
300
750
1100
300
450
900,1500
1900
clean charge
x
x
~x
x
x
x
Concentration
Low
High
template
1 µl (1:100)
15µl (1:100)
primer (11,12)
2.5 µl*2
dNTPs
1 µl
Pfu
0.5 µl
10xbuffer
5 µl
DMSO
1,25µl
H2O
36.25 µl
22.25 µl
sum
50µl
9-08-2010
eGFP
SV40PA=PCR6
ccdBamp
DNA
5µl=300ng
DNA
1µl=37,5ng
DNA
2µl=115ng
Buffer MC
2µl
Buffer D
2µl
Buffer H
2µl
BSA 1:10
2µl
BSA 1:10
2µl
BSA 1:10
2µl
EcoRI
0,5µl
XbaI
0,5µl
EcoRI
0,5µl
SpeI
0,5µl
PstI
0,5µl
PstI
0,5µl
H2O
13µl
H2O
14µl
H2O
13µl
sum
23µl
sum
20µl
sum
20µl
Ligation 1
eGFP
14,4µL
(144ng)
SV40PA
4,8µL
(48ng)
ccdBamp
2,9µL
(100ng)
T4 Buffer 10x
3µL
T4 Ligase
0,5µL
H2O
4,4µL
sum
30µL
Ligation at 22,5°C for 30 min, denaturation at 65°C for 10 min.
540ng
A260/A280=2,4
Transformation
9-09-2010
Plasmid extraction of ccdBcam, ccdBamp, Bak, CMV, PhiC31o
Plasmid
concentration [ng/µL]
A260/A280
ccdBcam
22.5
1.0
ccdBamp
42.5
1.2
Bak
17.5
0.8
CMV
10.0
0.7
PhiC310
60
1.4
PCRnr.
1
3
4a
4b
5
7a
9
10
template
pDS7 (1:100); 4µl
2a; 0.9µl+2b; 0.6µl
Bak; 0.5µl
Bak; 0.5µl
4a; 0.8µl+4b; 1µl
190-6 (1:100); 1µl
eGFP (1:25); 2µl
PhiC31o (1:10); 1µl
primer [10pmol/µl]
1,2; 2.5µl
3,6; 2.5µl
7,8; 2.5µl
9,10; 2.5µl
7,10; 2.5µl
13,14; 2.5µl
20,21; 2.5µl
22,23; 2.5µl
H2O
28µl
30.5µl
31.5µl
31.5µl
30.2µl
31µl
30µl
31µl
Annealing temperature
51.5°C
53.1°C
53.1°C
51.5°C
53.1°C
57.5°C
61°C
57.5°C
PCRnr.
1
4a
4b
5
7a
template
pDS7 (1:10); 1µl
Bak 1 ; 1µl
Bak 1; 1µl
4a, 4b; 2 x 1.5µl
pCT 190-6 (1:40); 1µl
primer
1, 2; 2 x 2.5µl
7, 8; 2 x 2.5µl
9, 10; 2 x 2.5µl
7, 10; 2 x 2.5µl
13, 14; 2x 2.5µl
H2O
31µl
31µl
31µl
30µl
30µl
Annealing temperature
52°C
52°C
48°C
50°C
55°C
9-10-2010
Agarose gel extraction of PCR 3, 4a, 5, 9, 10
PCR 3
PCR 4a
PCR 5
PCR 5*
PCR 9
PCR 10
concentration [ng/µl]
20
10
5
30
35
25
A260/A280
1.6
1.333
2.0
2.0
1.750
2.0
9-11-2010
9-12-2010
9-13-2010
name
4a-7
4a-8
4b-9
4b-10
3-3
3-6
6-11
6-12
DNA
4a; 2.4µl
4a; 2.4µl
4b; 0.5µl
4b; 0.5µl
3; 2µl
3; 2µl
6; 0.5µl
6; 0.5µl
primer [1pmol/µl]
7; 3.2µl
8; 3.2µl
9; 3.2µl
10; 3.2µl
3; 3.2µl
6; 3.2µl
11; 3.2µl
12; 3.2µl
Tris (10mM); pH 8.2
1.4µl
1.4µl
3.3µl
3.3µl
1.8µl
1.8µl
3.3µl
3.3µl
New PCR7a with Taq
template (~4ng)
2µl (1:100)
MasterMix for Taq
10µl
Primer *2
1.5µl *2
DMSO
0,5µl
H2O
4.5µl
sum
20µl
1: 94°C 2'
2: 94°C 30"
3: 64°C/62°C/60°C/58°C/56°C/54° 30"
4: 72°C 2'
5: (for each temperature)repeat 2-4 2x
6: 94°C 30"
7: 58°C 30"
8: 72°C 2'
9: repeat 6-8 29x
10: 72°C 10'
11: 15°C break
overnight culture inoculated of
New PCR7a with Phusion Hot Start
template
2ng
4ng
H2O
54µl
53µl
Buffer 5x
20µl
20µl
Primer (13,14)
2*10µl
2*10µl
DMSO
3µl
3µl
Hot Start
1µl
1µl
9-14-2010
bands 1 to 5: 2ng of template DNA
number:
7a diluted
7a undiluted
8
mastermix
50µl
50µl
10µl
primer
2*10µl
2*10µl
2*2µl
H2O
29µl
29µl
5µl
template
1µl 190-6 (1:100)
1µl 190-6
1µl 190-6 (1:100)
1
2
3
4
5
6
8
48°C
52°C
56,1°C
48°C
52°C
56,1°C
52°C
94°C 2'
94°C 30"
48°C/52°C/56,1°C 30"
72°C 1,5'
(for each temperature)repeat 30x
72°C 5'
9-15-2010
number
7a
7a
"8"
"8"
Mastermix
20µl
20µl
20µl
20µl
H2O
15µl
15µl
15µl
15µl
Primer
(13,14) 2*2µl
(13,14) 2*2µl
(13,16) 2*2µl
(13,16) 2*2µl
Template
190-6 (1:5) 1µl
190-6 (1:10) 1µl
190-6 (1:5) 1µl
190-6 (1:10) 1µl
program:
94°C 2'
94°C 30"
56°C/58°C 30"
72°C 1,5'
(for each temperature)repeat 30x
72°C 5'
Restriction Digestion of PCR 1 (17.8.), PCR 3 (10.9.), PCR 51 (10.9.), PCR 52 (10.9.), PCR 6 (17.8.), PCR 9 (10.9.), PCR 10 (30.8.) for ligation with vektor
1
3
51
52
6
9
10
H2O
13µl
12,5µl
5µl
13µl
13,5µl
13,5µl
12,5µl
Buffer H
2µl
2µl
2µl
2µl
2µl
2µl
2µl
BSA (1:10)
2µl
2µl
2µl
2µl
2µl
2µl
2µl
DNA
2µl
2,5µl
10µl
2µl
1,5µl
1,5µl
2,5µl
EcoR1
0,5µl
0,5µl
0,5µl
0,5µl
0,5µl
0,5µl
0,5µl
Pst1
0,5µl
0,5µl
0,5µl
0,5µl
0,5µl
0,5µl
0,5µl
concentration (ng/µl)
A260/A280
(supposed) lenght
1
20
1,000
492
3
5
1,000
1087
51
22,5
1,8
688
52
7,5
1,5
688
6
10
1,333
237
9
20
1,6
808
10
17,5
1,75
1888
for
#
backbone
insert (µl)
charge (µl)
Buffer 10x (µl)
Hsub<2</sub> (µl)
100ng
1
4
7,12
20
2
6,38
50ng
3
2
31,48
40
4
2,02
100ng
51
4
8,85
20
2
4,65
100ng
52
4
26,56
40
4
4,94
100ng
6
4
6,86
20
2
13,36
100ng
9
4
11,7
20
2
1,8
100ng
10
4
31,24
40
4
4,26
new PCR 7a & "8"
7a-1
7a-2
"8"-1
"8"-2
mastermix (µl)
10
10
10
10
template
190-6 1µl
190-6 2µl
190-6(1:10) 1µl
190-6(1:10) 2µl
primer
13&14 2*1µl
13&14 2*1µl
13&16 2*1µl
13&16 2*1µl
H2O
7µl
6µl
7µl
6µl
94°C 2'
94°C 30"
56°C 30"
72°C 1,5'
(for each temperature)repeat 30x
72°C 5'
12°C forever
9-16-2010
1
3
5
6
9
10
template
prev TRE(1:30) 1µl
2a(1:5) 1µl + 2b 0,5µl
4a 0,5µl + 4b 0,5µl
SV40PA (pcDNA3)(1:50) 1µl
eGFP(1:5) 1µl
PhiC310(1:6) 1µl
primer
1&2 2*2,5µl
3&6 2*2,5µl
7&10 2*2,5µl
11&12 2*2,5µl
20&21 2*2,5µl
22&23 2*2,5µl
Tm
54°C
50°C
49°C
56°C
58°C
58°C
mastermix (µl)
25
25
25
25
25
25
DMSO
1,25µl
1,25µl
1,25µl
1,25µl
1,25µl
1,25µl
H2O (µl)
17,75
17,25
17,75
17,75
17,75
17,75
94°C 2'
94°C 30"
Tm (see above) 30"
72°C 1,5'
(for each temperature)repeat 30x
72°C 5'
9-17-2010
1
3
5
6
9
10
pellet
no
yes
yes
yes
yes
yes
yes
100µl
no
yes
yes
yes
yes
yes
yes
1
5
6
template
pTRE Rev 0,5µl
4a 1,5µl + 4b 1,5µl
pcDNA3 0,5µl
primer
1&2 2*2,5µl
7&10 2*2,5µl
11&12 2*2,5µl
buffer 10x
5µl
5µl
5µl
dNTPs
1µl
1µl
1µl
Pfu
0,5µl
0,5µl
0,5µl
H2O
26,75 µl
24,25 µl
26,75 µl
T<sub<m</sub>
51°C
50°C
55°C
9-18-2010
9-19-2010
9-20-2010
9-21-2010
9-22-2010
9-23-2010
9-24-2010
9-25-2010
9-26-2010
9-27-2010
9-28-2010
9-29-2010
9-30-2010
10-01-2010
10-02-2010
10-03-2010
![]()
![]()

 "
"