Team:Heidelberg/Project/miRNA Kit
From 2010.igem.org
(→raw data) |
(→advancement) |
||
| Line 114: | Line 114: | ||
* achievement of specific target cell [https://2010.igem.org/Team:Heidelberg/Project/Capsid_Shuffling tropism] | * achievement of specific target cell [https://2010.igem.org/Team:Heidelberg/Project/Capsid_Shuffling tropism] | ||
→ further improvement of gene expression tuning | → further improvement of gene expression tuning | ||
| - | + | <br/> | |
| + | <br/> | ||
| + | <br/> | ||
| + | === tuning raw data === | ||
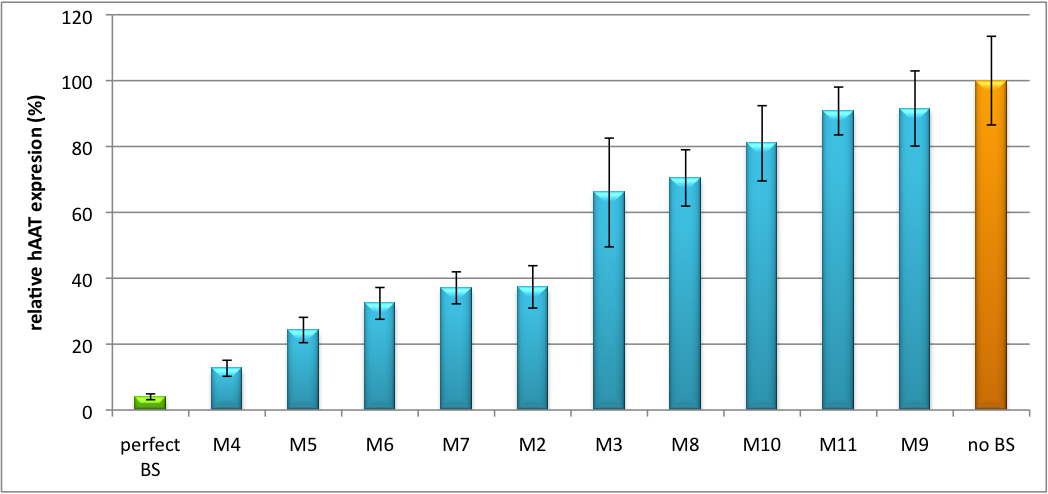
| + | For our <i>in vitro</i> tuning, you can have a look even at our unprocessed data with specific [https://2010.igem.org/Team:Heidelberg/Notebook/Methods#nomenclature nomenclature] | ||
| + | [[Media:Plate1 process H1.xls]], <br/> | ||
| + | [[Media:Plate2 process H1.xls]], <br/> | ||
| + | [[Media:Plate3 process H1.xls]], <br/> | ||
| + | [[Media:Haat 20101022 M1-M4 ctrl H1.xls]], <br/> | ||
| + | [[Media:Haat 20101026 M5-M8 ctrl H1.xls]], <br/> | ||
| + | [[Media:Haat 20101026 M9M22 ctrl H1.xls]], <br/> | ||
| + | [[Media:HAAT H1 final.xls]] <br/> | ||
| + | [[Media:Plate1 process U6 haat.xls]],<br/> | ||
| + | [[Media:Plate2 process U6 haat.xls]],<br/> | ||
| + | [[Media:Plate3 process U6 haat.xls]],<br/> | ||
| + | [[Media:Haat 20101026 plate2 U6.xls]],<br/> | ||
| + | [[Media:Haat 20101026 plate1 U6.xls]],<br/> | ||
| + | [[Media:Haat 20101026 plate3 U6.xls]],<br/> | ||
| + | [[Media:HAAT U6 final.xls]].<br/> | ||
{{:Team:Heidelberg/Bottom}} | {{:Team:Heidelberg/Bottom}} | ||
Revision as of 06:11, 27 October 2010

|
|
||
 "
"