Team:Heidelberg/Project/miRNA Kit
From 2010.igem.org
(→Advancement) |
(→Advancement) |
||
| Line 44: | Line 44: | ||
=== Advancement === | === Advancement === | ||
* digestion of miR Kit construct with BamHI | * digestion of miR Kit construct with BamHI | ||
| - | * cloning into viral backbone (e. g. [ | + | * cloning into viral backbone (e. g. [https://2010.igem.org/Team:Heidelberg/Notebook/Material pBS_U6]) |
* [https://2010.igem.org/Team:Heidelberg/Notebook/Methods#Virus_Production virus production] | * [https://2010.igem.org/Team:Heidelberg/Notebook/Methods#Virus_Production virus production] | ||
* infection of cells | * infection of cells | ||
Revision as of 23:43, 27 October 2010

Synthetic miRNA Kit
miTuner - a kit for microRNA based gene expression tuning in mammalian cells
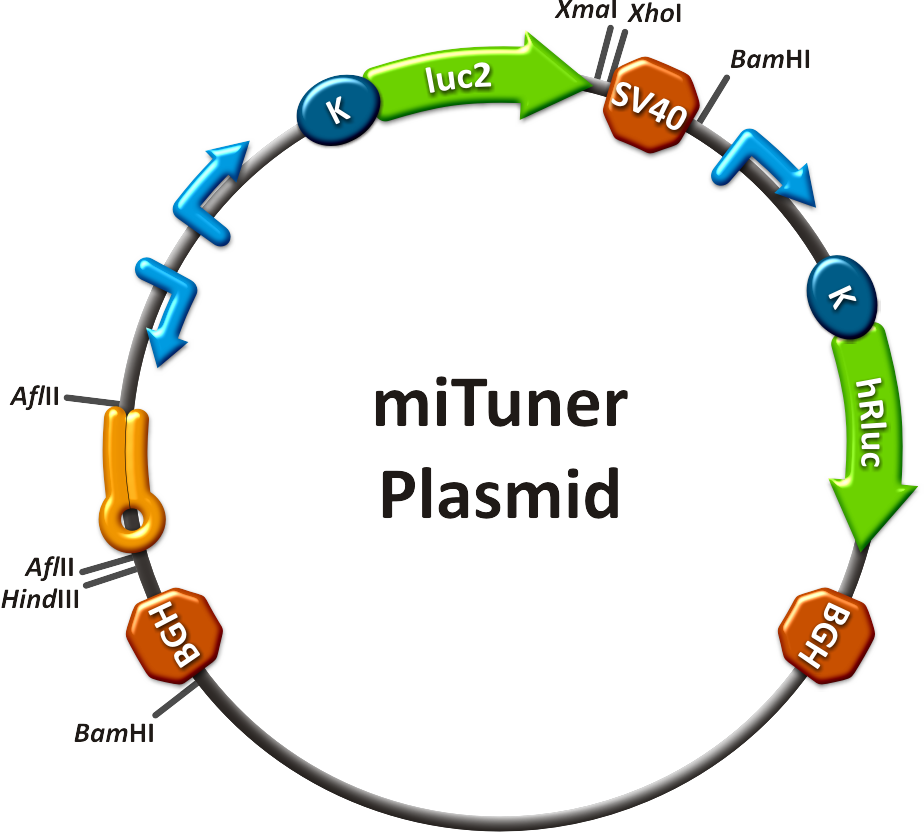
to plan, conduct and evaluate experiments dealing with miBricks (i. e. microRNA related Biobricks) as key regulators in mammalian cells.AbstractRegulation of any gene of interest has never been as easy as with our miRNA-based expression tuning kit miTuner. Rational design of synthetic miRNA binding sites according to our recommendations enables fine-tuning of gene expression in a range between 5% and 100%. Additionally, we offer Off- and On-targeting switches which effect GOI expression in only one or all but one tissue depending on endogenously occurring miRNAs. The tuning application is based on a dual promoter construct that expresses a GOI controlled by a synthetic miRNA which is expressed from the same construct (figure 1a). Differing miRNA-binding site interaction efficiencies caused by binding sites of different sequence properties are used to distinctly adjust expression strength of the GOI. For Off-targeting, the GOI is under control of miRNAs that are found in tissue where gene expression is thereupon silenced while the GOI can still be expressed in other tissues as visualized in figure 1b. On-targeting is based on the expression of the GOI from a promoter containing a Tet Operon that negatively regulates gene expression in the presence of a Tet Repressor (figure 1c). If the Tet Repressor is under control of perfect binding sites for endogenous miRNAs , it will be downregulated in the target tissue, releasing the promoter and enabling specific GOI expression. IntroductionMicroRNAs (miRNAs) are short endogenous, non-coding RNAs that mediate gene expression in a diversity of organisms [http://2010.igem.org/Team:Heidelberg/Project/miRNA_Kit#References (Bartel, 2004)]. Although the understanding of their biological functions is progressing remarkably, the exact mechanisms of regulation are still not unambiguously defined. However, it is commonly believed that miRNAs trigger target mRNA regulation by binding to 3’ untranslated region (UTR) of its target [http://2010.igem.org/Team:Heidelberg/Project/miRNA_Kit#References (Chekulaeva and Filipowicz, 2009)]. Exact principles of expression knockdown mediated by miRNA are still in debate [http://2010.igem.org/Team:Heidelberg/Project/miRNA_Kit#References (Eulalio et al., 2008)]. miTuner Kit componentsThe miTuner Kit consists of three basic components:
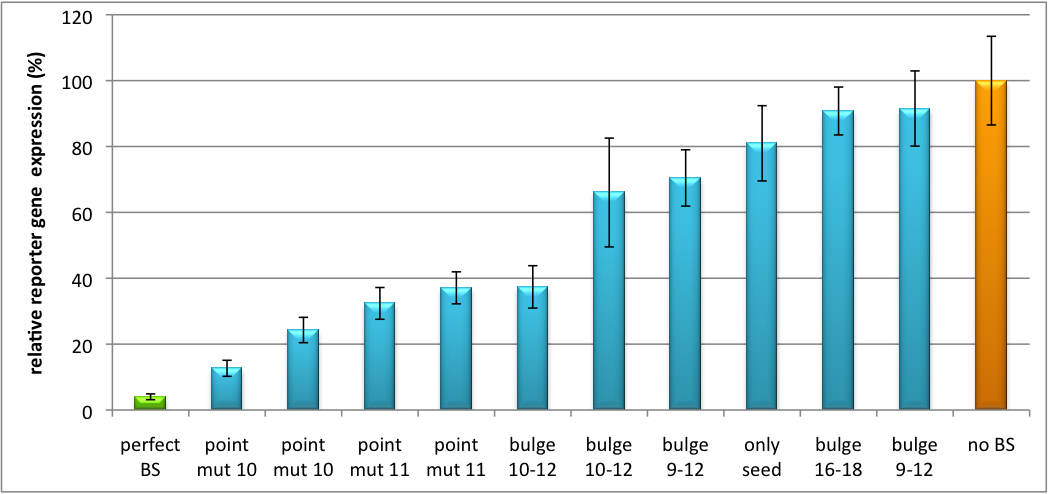
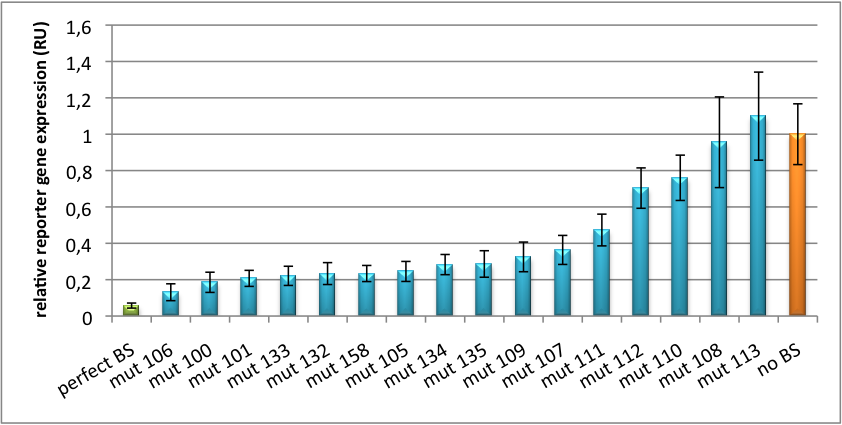
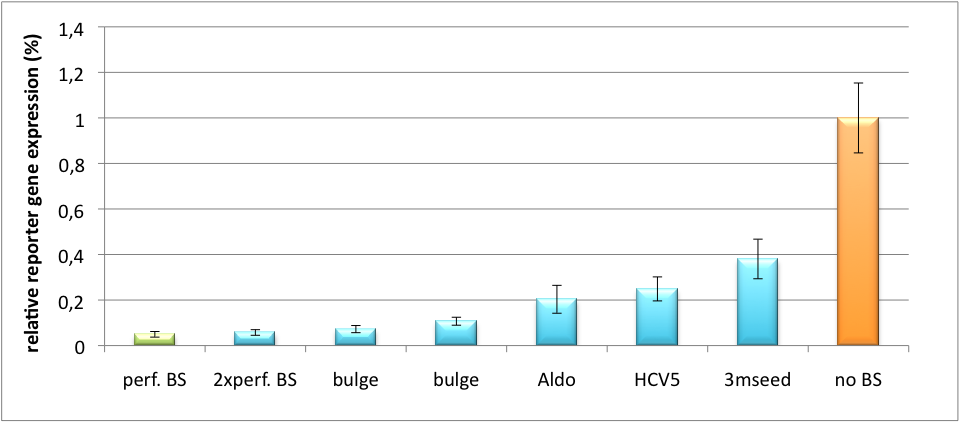
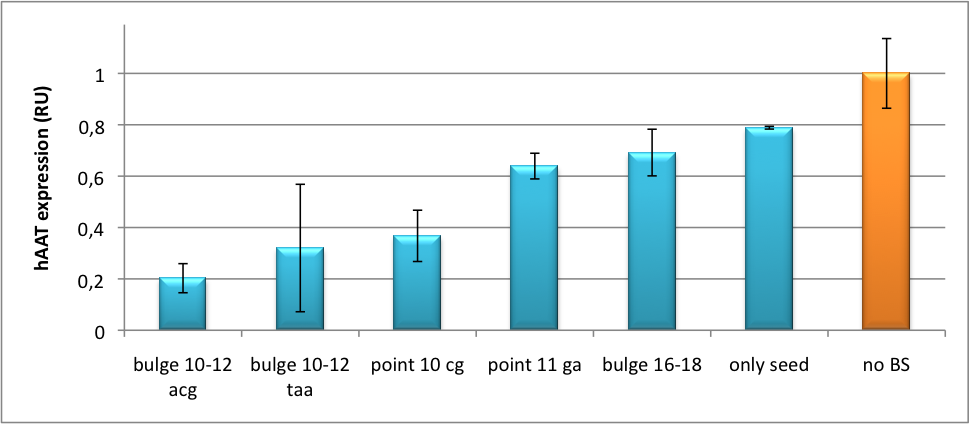
Please find further information about the kit components and engineering of the kit here. ResultsAll gene regulatory constructs for tuning, Off- or On-targeting can easily be assembled using BBB standard cloning from our miRNA Kit parts. After successful cloning, the constructs can be transfected onto a cell line of choice or transferred into a virus backbone for in vivo experiments. For our proof of principle, we used firefly luciferase normalized to Renilla luciferase on miTuner to characterize knockdown efficiencies of different binding sites and show Off- and On-targeting by mouse infection carried by an AAV virus. miTuner: Expression Fine-Tuning by Synthetic miRNAsThe data shows a precisely tuned expression from almost 0% to 100% (Fig. 2, Fig. 3). Lowest expression refers to complete knockdown through fusion of perfect binding sites (always green bar on the left hand side of the figures) to the reporter gene. Expression from a construct without binding sites is set as 100% (always orange column on the right hand side of the figures). In presence of the specific shRNA miR, gene expression was mediated to various levels through interactions with the different imperfect binding sites. Whereas, when an unspecific shRNA miR was expressed, gene expression remained unaffected (see raw data below). This reference shows that the binding sites were correctly designed, since they seem to interact specifically with a referring shRNA miR. Figure 2 shows the results of Dual-Luciferase measurements of the miTuner plasmid with binding sites against shhAAT behind firefly luciferase. The highest knockdown can be achieved by using a perfect binding site. Single mutations outside the seed region at position 11, 12 or 10-12 lead to knockdown between 10% and 60% compared to unregulated expression. Bulges close to the seed region or changes in the seed region itself lead to very low downregulation. Having only the seed region as a target for the miRNA also leads to a less efficient knockdown compared with binding sites containing flanking regions. Figure 3 shows the same assay using binding sites against shhAAT within the pBS U6 vector. The results are overall similar, with changes in or directly adjacent to the seed region having the highest impact on knockdown efficiency. The measurement uses the same binding sites as the one conducted in pBS U6, just having a H1 promoter instead of U6 promoter. We further analyzed binding sites derived from miR122 in the dual luciferase vector PsiCheck2 as can be seen in figure 4. Here we tested sixteen mutated binding sites in order to observe minute fine-tuning between one binding site and the next. Mutated Binding sites 123, 133, 134, 135 and 158 contain 4bp-bulges (non-paired regions) that don not seem to diminish knockdown efficiency much. 107 contains one binding site, while 134 and 135 contain two binding sites for the same miRNA and show a stronger knockdown than 107. Off-Targeting Using Endogenous miRNAAnother application of our synthetic miRNA Kit profits of tissue specific endogenous miRNAs expression. These can be exploited for either Off- or On-Targeting. To enable Off-Targeting, the GOI expressed on miTuner can be tagged with a miRNA binding site specific for one or a combination of endogenous miRNA of the tissue that is to be excluded from gene expression. In our experiment, we transfected Huh7 (human hepatoma) cells that endogenously overexpress miR122 with the miTuner construct after cloning different variations of binding sites for miR122 behind firefly luciferase. Figure 5 shows the results of the dual luciferase assay. Perfect binding sites result in almost complete inhibition of expression.
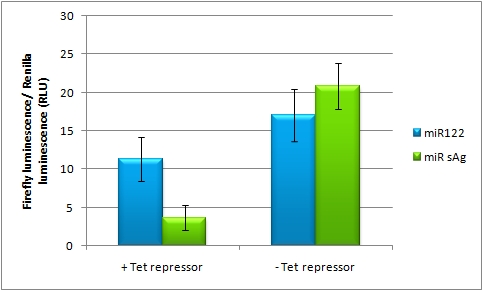
On-Targeting Using Endogenous miRNAIn line with the Off-targeting approach, In the case of On-targeting the presence of a certain miRNA in a cell switches on expression of the GOI. This can be accomplished by using a repressor that is targeted by an endogenously expressed miRNA. We exemplified this scenario by using a Tet Repressor fused with a perfect binding site for miRNA 122, a liver-specific miRNA (Jopling et al., 2005). At the same time, the promoter expressing the GOI would be under control of a Tet Operator. Upon presence of the miRNA 122, the Tet Repressor would be knocked down, release the promoter and expression of the GOI could be established.
DiscussionStrikingly, the order of constructs in terms of knockdown for the imperfect binding sites is similar. M4, M5 and M6 always show strong knockdown, whereas M9, M10 and M11 show only loose down-regulation. Consulting the binding site sequences, the weak knockdown can be addressed to bulges in the supplementary region or to complete lack of the 3' region of the binding site. Still high strength could be maintained due to only single nucleotide exchanges in the central region of the binding site. The principle of smooth regulation was also demonstrated for miR122, a microRNA that is exclusively upregulated in hepatic cells. Referring binding sites were cloned into psiCHECK-2 backbone (Promega) and due to sequence mutations different Luciferase levels were detected again (Fig. 3).
Application of miTunerIn Vitro Regulation of a Therapeutic Gene, hAATWe further tested our kit using a gene that is an interesting candidate for gene therapy, i. e. human alpha-1-antitrypsin (hAAT). Tight control of the genetic activity is fundamental, since deficiencies of hAAT can cause emphysema [http://2010.igem.org/Team:Heidelberg/Project/miRNA_Kit#References (Lu et al., 2006)]. With our tuning kit we have a powerful mean at hand to mediate expression levels. In this approach, we tagged hAAT, that we used as our GOI, with binding sites (for miRsAg) that we measured and characterized with our miMeasure construct beforehand (data not shown). There is some evidence, that the principle works also with this therapeutic gene in HeLa cells (fig. 7). This is a first potential therapeutic approach applying ELISA for measurements. It is obvious: different binding sites result in different knockdowns of gene expression. Some imperfect binding sites - e. g. single seed region - indicate even similar expression levels in accordance to the figures shown before. It can be stated, that the tuning idea seems to work for attempts varying in applied miRNAs, binding sites and reporter genes. The hAAT as a GOI is worth testing because it is mainly secreted in liver -our target tissue of choice. Efficient transduction can be accomplished by infection with selected viruses. Dealing with hAAt intertwines our two approaches of specific gene therapy, therefor being a relevant field for future research. In Vivo ValidationThe constructs were tested in two different backbones: pBS_U6 and pBS_H1. Both are in viral context, meaning that they contain inverted terminal repeats (ITRs). The constructs can be packed into the capsid of an adeno-associated virus (AAV). Those constructs we also chose for virus production to infect cells even more efficiently as compared to transfections. Because of the significant data, we decided to inject the viruses into mice to see the tuning effect also in vivo. The pBS_H1 construct should be preferred for mice injections since the expressed synthetic shRNA miR against human alpha-1-antitrypsine (shhAAT) is cytotoxic in higher concentrations. The pBS_H1 backbone leads to moderate expression ranges, still obviously showing the tuning effect. ModelingAfter creating a binding site library and testing the miRNA-binding site interaction in vitro, we were able to compute an in silico model based on a machine learning approach to predict knockdown efficiencies. A more detailed description of the different binding sites, we characterized can be found in our measurements page. MethodsmiTuner: Expression Fine-Tuning by Synthetic miRNAsThe miTuner was assembled out of different parts. Cloning was done following standard protocols. Regulation of gene expression can be achieved by fusing miRNA binding sites right behind a GOI. In case a referring shRNA miR is expressed, the GOI is knocked down. Strength of regulation thereby depends on binding site properties. We are able to tune gene expression linearly over a broad range. This is a first proof of principle for various miRNA-mRNA interactions in vitro. Therefore, we transfected HeLa cells in principle with our [http://partsregistry.org/Part:BBa_K337036 pSMB_miTuner Plasmid HD3]. It turned out, that there was no obvious effect of different binding sites on reporter gene expression (data not shown). We assume that the RSV driving the shRNA miR is too weak for tight regulation of the referring binding site behind the GOI which is driven by the very strong CMV promoter. Only if a sufficient amount of shRNA miR binds to its target, translation is significantly repressed. Thus, we expressed the shRNA miR from a separate plasmid which was always co-transfected with the original tuning construct. The reporter genes - i. e. hFluc and hRluc - were also expressed from separate plasmids to get a reference as well as a transfection control. Then, we conducted a Dual Luciferase Assay for quantification of gene expression. On- and Off-TargetingMeasurements were done in HeLa cells overexpressing miR122 from plasmid. Besides that, even endogenous miR122 levels were sufficient for off-targeting HuH cells (Fig. 4). A single perfect binding site leads to 95% knockdown, which seems to be maximum, since even a perfect binding site duplicate results in the same reporter gene expression.
References
|
||||||||||||||||||||||||||||||||||
 "
"