Team:Caltech/Week 7
From 2010.igem.org
(Difference between revisions)
| (4 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
==Monday 7/26== | ==Monday 7/26== | ||
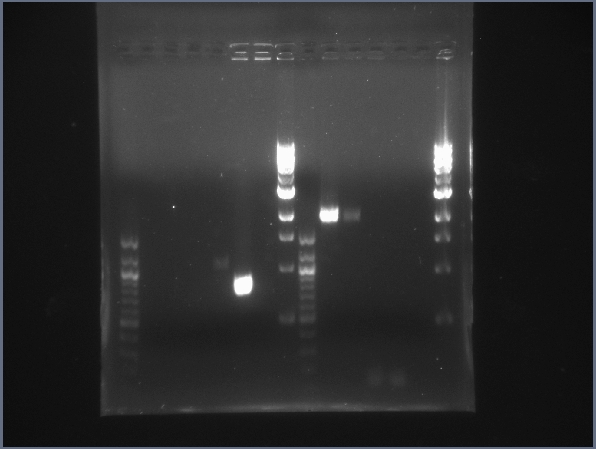
| + | [[Image:CIT-cpcr-gel1-7-26.jpg|200px|thumb|CPCR gel featuring L16, L19, L23, L24, L31 & L34. (7/26)]] | ||
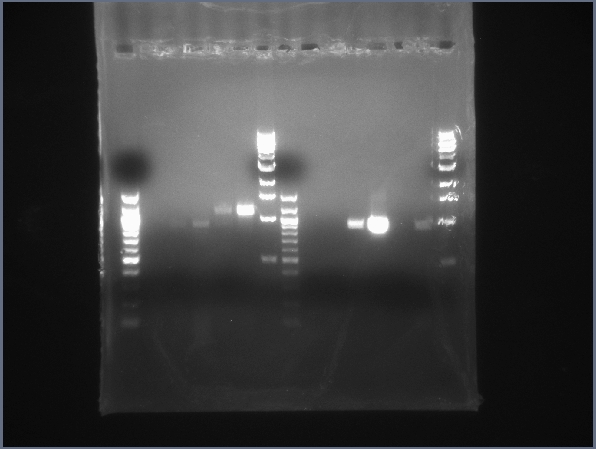
| + | [[Image:CIT-cpcr-gel2-7-26.jpg|200px|thumb|CPCR gel featuring L34, L35, L40, L42, L43 & L44. (7/26)]] | ||
| + | * Started PCR reaction to extract the HSP (K112400) from its Berkeley Standard backbone (BBb). (Used same PCR parameters as CPCR from 7/22. | ||
| + | * Group meeting! | ||
| + | ** By next week: Need to design test-construct experiments, as they will be ready sometime early next week. | ||
| + | *** Can use PCR machine to achieve easy temperature gradients | ||
| + | *** Use spectrophotometer and eventually microscope to assay fluorescence. | ||
| + | *** Consider a plate assay? | ||
| + | ** AIBN issues: | ||
| + | *** Consider using low-concentration DMSO to help dissolve AIBN in LB/cell mixture. | ||
| + | *** Need to figure out how much unreacted (or otherwise) AIBN remains after mixing with cells. | ||
| + | *** Need to test crosslinking using hydrolyzed and epoxidized oil mixed with lysate and with cells to tweak concentration of AIBN needed, and to see what it makes. | ||
| + | ** TGase: | ||
| + | *** purchase urea? | ||
| + | *** To prevent a solid plug forming upon addition of TGase powder to sample, prepare liquid stock of concentrated TGase. | ||
| + | ** Wiki: | ||
| + | *** Should visit biofab.org for research material for human impact segment. | ||
| + | ** In general, we should set goals for both the end of SURF and for the end of the summer and determine what we need to do to get on track for them. | ||
| + | *** Printing in 2D | ||
| + | *** Working AND gate, at least in bulk fluid | ||
| + | *** Characterization of 3D hydrogel/plastic | ||
| + | *** Crosslinking data | ||
| + | * PCR purified K112400 PCR product & digested as plasmid insert. | ||
| + | * Transformed RFP bricks of verious resistances to use as backbone for future ligations | ||
| + | * Ran 2 gels of today's CPCR products. | ||
==Tuesday 7/27== | ==Tuesday 7/27== | ||
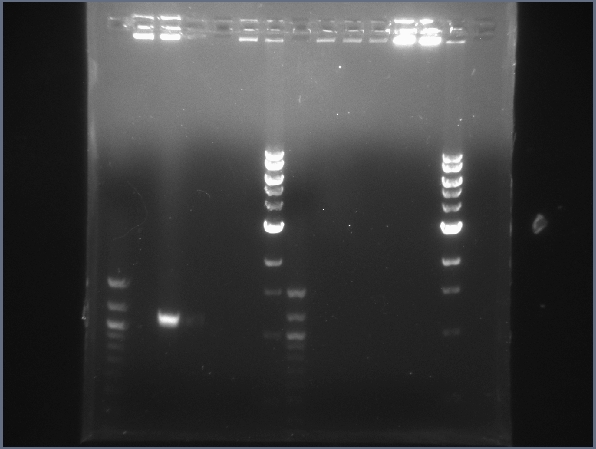
| + | [[Image:CIT-cpcr-gel-7-27.jpg|200px|thumb|CPCR gel featuring L16, L19, L31, L34, & L35. (7/22)]] | ||
| + | * Began more CPCR on failed ones from yesterday: L16, L19, L31, L34, L35 | ||
| + | * Began more digests of miniprepped DNA from K112400 | ||
| + | * Made more electrocompetent cells. Began two test transformations. | ||
| + | * Transformed ligation products: L42, L46, L47 and the HSP. | ||
| + | * Began 11 new ligations: | ||
| + | ** L48: R0082 + I13504 + Cm | ||
| + | ** L31: L17 + K215000 + Kan | ||
| + | ** L34: L17 + L21 + Amp | ||
| + | ** L35: L18 + L21 + Amp | ||
| + | ** L22: K156012 + B0034 + Cm | ||
| + | ** L24: K156014 + B0015 + Cm | ||
| + | ** L20: C0077 + B0015 + Cm | ||
| + | ** L49: K112400 + L40 + Cm | ||
| + | ** L25: B0015 + M30109 + Tet | ||
| + | ** L26: R0077 + K124017 + Cm | ||
| + | ** L50: L18 + L27 + Amp | ||
| + | * Note: Need to upstream-digest more of: J23119, L17, K156014, B0015 for future ligations. | ||
==Wednesday 7/28== | ==Wednesday 7/28== | ||
| + | * Began CPCR reactions on the HSP, L42, L46, L48. | ||
| + | * Transformed all ligations from yesterday | ||
| + | * Re-ran gel from yesterday | ||
| + | ** Same results: looks like 19-4 is a success | ||
| + | * Ran gel of today's CPCR to determine if the HSP ligations worked. | ||
| + | ** All bands appear to have a 100-400bp smear and no other bands, making the results indeterminate. | ||
==Thursday 7/29== | ==Thursday 7/29== | ||
| + | * Re-did the following ligations: L16, L31, L34, L35, HSP (in pSB1C3, for submission), L42, L46, L47 | ||
| + | * Started epic amounts of colony PCR: | ||
| + | ** 3 colonies of each: L42, L46, L31, HSP-Amp, L35, L50, L34, L48, L24, L49, L22, L20 | ||
| + | ** Gels for each are included to the right -> | ||
| + | ** Successful: L42-4/-5, L46-4/-5, L47-4, HSP-Amp-5/-6 | ||
==Friday 7/30== | ==Friday 7/30== | ||
| + | * Prepped 47-4 (HSP + B0034), L42-4/-5, L46-4/-5 (HSP + R0077), HSP-Amp-5/-6 | ||
| + | * Began a ton of digests: | ||
| + | ** 2x Tet backbone | ||
| + | ** U19: K156012 (us) | ||
| + | ** U20: K156014 (us) | ||
| + | ** U21: C0077 (us) | ||
| + | ** U22: L19 (us) | ||
| + | ** U23: L46 (us) | ||
| + | ** U24: L47 (us) | ||
| + | ** U25: L17 (us) | ||
| + | ** D17: B0034 (ds) | ||
| + | ** D18: B0015 (ds) | ||
| + | ** D19: L18 (ds) | ||
| + | ** D20: L19 (ds) | ||
| + | ** D21: HSP-Amp (ds) | ||
==Weekend 7/31-8/1== | ==Weekend 7/31-8/1== | ||
| + | === Saturday 7/31 === | ||
| + | * Transformed L22, L24, L33, L43 | ||
| + | ** Failed: L51, L52, L53, L25 (cuvette arced) | ||
| + | * Ran gel of M30109 CPCR product: no bands! | ||
| + | ** Is M30109 incorrect? | ||
| + | |||
| + | === Sunday 8/1 === | ||
| + | * Removed transformation plates: L22 & L24 were successful; L33 & L53 failed. | ||
| + | |||
| | ||
}} | }} | ||
Latest revision as of 01:05, 4 August 2010
|
People
|
Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | Week 11 | Week 12 | Week 13 | Week 14 | Week 15
Monday 7/26
Tuesday 7/27
Wednesday 7/28
Thursday 7/29
Friday 7/30
Weekend 7/31-8/1Saturday 7/31
Sunday 8/1
|
 "
"