Team:Tokyo Tech/Project/wolf coli
From 2010.igem.org
| Line 4: | Line 4: | ||
<div id="tf_menu"> | <div id="tf_menu"> | ||
menu | menu | ||
| - | [[Team:Tokyo_Tech/Project/wolf_coli/ | + | [[Team:Tokyo_Tech/Project/wolf_coli/New_Series_of_PompC|PompC]] |
| - | [[Team:Tokyo_Tech/Project/wolf_coli/ | + | [[Team:Tokyo_Tech/Project/wolf_coli/lacIM1|lacIM1]] |
</div> <!-- tf_menu --> | </div> <!-- tf_menu --> | ||
Revision as of 18:30, 25 October 2010
Contents |
Wolf coli
Introduction
In order to assemble a more intelligible& imaginable system, we linked our project to a well-known character ”Wolfman”. In our attempt to produce “wolfcoli”, we introduced the Artificial Cooperation System. A red-light-dependent gene expression system and synthetic band detector circuit were used to perform function designed.
Highlight
・In order to design E. coli to have humanity, we attemped to integrate a red-light-dependent gene expression system and synthetic band detector circuit into the Artificial cooperation system.
・We have constructed the NEW BioBrick series of ompC promoter .
-We found that lacIM1 shows weaker repression than WT (Fig. 11).
・We have characterized lacIM1(BBa_K082026) which is the key component in the synthetic band detector circuit. Though this part was registered by USTC(2008), it was not well characterized in Wiki.
-We found that lacIM1 shows weaker repression than WT (Fig. 11).
Requirement
Red-light-dependent gene expression system
A red-light-dependent gene expression system has been introduced into E. coli(Anselm Levskaya et al.2005). And these BioBrick parts have been registered.
Photoreceptors are not found in E. coli. Then, they introduced a light sensor form a cyanobacterium into E. coli. The response regulator of phytochrome do not directly regulate gene expression, so they fused a cyanobacterial photoreceptor from Cph1 to an E. coli intracellular histidine kinase domain and response-regulator from EnvZ–OmpR. Moreover , Cph1–EnvZ chimaeras were then activated by introduction of two phycocyanobilin-biosynthesis genes that convert heme into phycocyanobilin.
In low red light condition, Cph1–EnvZ chimeras are activated. EnvZ is autophosphorylated and passes phosphoryl group intramolecularly to OmpR. Then, phosphorylated OmpR binds to ompC promoter and activates the transcription of the downstream gene.
In high red light condition, Cph1–EnvZ chimaeras are not activated. EnvZ is dephosphorylated, and thus result in no phosphorylation of OmpR. Then, OmpR can’ t bind to ompC promoter. The transcription of the downstream gene aren’t occur.
What is important in this system is that light intensity is converted into concentration of phosphorylated OmpR.
Synthetic band detector circuit
Gene expresses only within the specific concentration range of chemical signals. Synthetic band detector circuit exhibits transient gene expression in response to concentration of chemical signals. (Subhayu Basu et al.2005) Previously, USTC(2008) attempted to build this circuit and registered parts for this circuit.
In Basu’s group paper, LuxR, an AHL-dependent transcriptional regulator, was used. (Fig. 2) LuxR activates the expression of cI and LacIM1, which has a lower affinity to lac promoter than WT. Therefore, LacIM1 shows weaker repression than WT.
High concentration of AHL(Fig. 3a) results in high levels of CI and LacIM1 and repression of GFP.
At low concentration of AHL(Fig. 3b), Lac IM1 and CI are expressed only at basal levels. This enables the expression of a LacIWT, again resulting in GFP repression
At intermediate concentration of AHL (Fig. 3c), it’s result in moderate levels of CI and LacIM1. However, because the repression efficiency of CI is significantly higher than that of LacIM1, CI effectively shuts off LacI expression while the LacIM1 concentration is below the threshold required to repress GFP production. This difference between the CI and LacIM1 repression efficiencies, in combination with a feed-forward loop that begins with LuxR and culminates in GFP, affords the circuit the desired non-monotonic response to AHL dosages.
Genetic circuit
circuit
We aimed to introduce a red-light-dependent gene expression system and synthetic band detector circuit into the Artificial Cooperation System. (Fig. 4) Two systems were united by applying OmpR. OmpR usually works as regulative factor of red-light sensing system, and we utilized this to regulate synthetic band detector circuit. OmpR is a transcriptional regulator, which activates the expression of lambda repressor (CI) and Lac repressor (LacIM1, a product of a codon-modified lacI) in this system
・At night (Low light intensity) In the dark, high concentrations of phosphorylated OmpR activates ompC promoter. That’s results in high cytoplasmic levels of CI and LacIM1 and repression of the inverter. Then, Artificial Cooperation System turns on, too.
・At daytime (High light intensity) Light drives the sensor to a state in which phosphorylation of OmpR is inhibited. There is no expression from ompC promoter. In low concentrations of phosphorylated OmpR, LacIM1 and CI are expressed only at basal levels. This enables the expression of a wild-type LacI, again resulting in repression of the inverter. Then, Artificial Cooperation System turns on , too. ・At full moon night (Intermediate light intensity) Intermediate phosphorylated OmpR concentration results in moderate levels of CI and LacIM1. However, because the repression efficiency of CI is significantly higher than that of LacIM1, CI effectively shuts off LacI expression while the LacIM1 concentration is below the threshold required to repress the inverter. This difference between the CI and LacIM1 repression efficiencies drives the inverter to a state of turning on. It’s results in turning off the Artificial Cooperation System.
・Seriese of PompC In our system, transient gene expression need to occur only at light intensity of full-moon. Therefore, we must find an appropriate range of the light intensity. To solve this problem, we have the following approachs. Ⅰ Modification of the chimaera protein(sensor protein, cph8) alters the ratio of phosphorylated OmpR, which is the transcriptional activator of the red-light dependent gene expression. Ⅱ Modification of OmpR protein also alters the ratio of phosphorylated OmpR, which is the transcriptional activator of the red-light dependent gene expression. Ⅲ Modification of ompC promoter alters the binding efficiency of phosphorylated OmpR.
As written above, there are various methods to integrate the two systems. Among the approaches, modification of the Chimera protein and OmpR protein is very difficult. Therefore, we made the series of ompC promoter. 実験、詳細については↓で行ったので見ていってね。
・LacIM1
Intermediate phosphorylated OmpR concentrations result in moderate levels of CI and LacIM1. However, because the repression efficiency of CI is significantly higher than that of LacIM1, CI effectively shuts off LacI expression while the LacIM1 concentration is below the threshold required to repress the inverter. This difference between the CI and LacIM1 repression efficiencies can afford the circuit the desired non-monotonic response to phosphorylated OmpR concentration. Therefore, lacIM1 is a key component for building the synthetic band detector circuit. We characterize the function of this part (BBa_K082026) , which was designed by USTC (2008) .
実験、詳細については↓で行ったので見ていってね。
Works
Overview of new OmpC promoter series
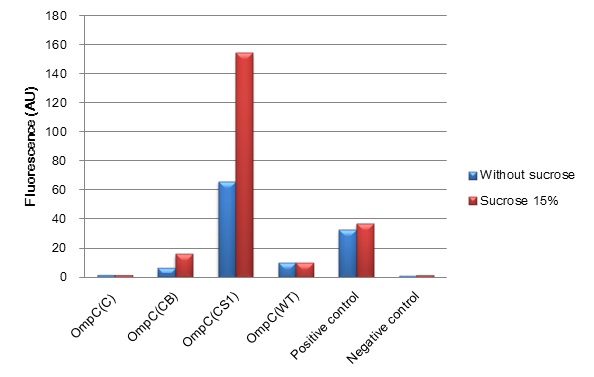
We constructed BioBrick parts of the new series of osmoregulative promoters which are derivatives of the wild type OmpC promoter. The new series of promoters are PompC(C), PompC(CB) and PompC(CS1). In order to measure the strength of each promoter, we used GFP as a reporter. We found that expression of GFP in OmpC(CB) and OmpC(CS1) promoters increased in high osmolarity medium comparing with the expression in low osmolarity medium. In contrast, under same conditions, there is no significant difference of GFP expression in OmpC(C) and OmpC(WT) promoters.
- New BioBrick Parts
We succeeded in designing 2 news osmoregulative promoters, POmpC(CB) and POmpC(CS1), which can also be utilized in light sensitive system.
...see more about PompC
Overview of characterozation of LacIM1
LacIM1 is a key component in the synthetic band detector circuit. Thus, we characterize the function of this part (BBa_K082026), which is designed by USTC(2008) . In order to measure the function of lacI proteins, we constructed following two plasmids, BBa_K395401, and BBa_K395402, which have an arabinose inducible promoter(Fig. 10). We measured GFP expression dependent on the input of arabinose and IPTG. For assay, we introduced two plasmids into DH5α. LacI proteins produced by arabinose induction represses GFP expression. The repression by lacI prorteins can be inhibited by IPTG induction.
Results : We found that lacIM1 shows weaker repression than WT (Fig. 11). This is the result of LacIM1. In the absence of both arabinose and IPTG, GFP expression is about 1.8-folds stronger than that of the presence of arabinose and the absence of IPTG. We found that GFP expression is dependent on product of lacIM1 by arabinose induction. When there was LacIM1 by arabinose induction, GFP expression was repressed. That’s result shows that this repression is effect of LacIM1. This repression is dependent on product of lacIM1 by arabinose induction Therefore, This shows that this repression is the function of LacIM1. Increase of GFP production by addition of IPTG, with or without arabinose induction, shows the repressions are dependent on LacIM1. Without arabinose, increase of GFP production by addition of IPTG is leaky expression of LacIM1. This leaky expression could also explain the result of LacWT.(See more…)
 "
"