Team:Tokyo Tech/Project/wolf coli/lacIM1
From 2010.igem.org
4-2 lacIM1 for band-detect network
Contents |
Abstract
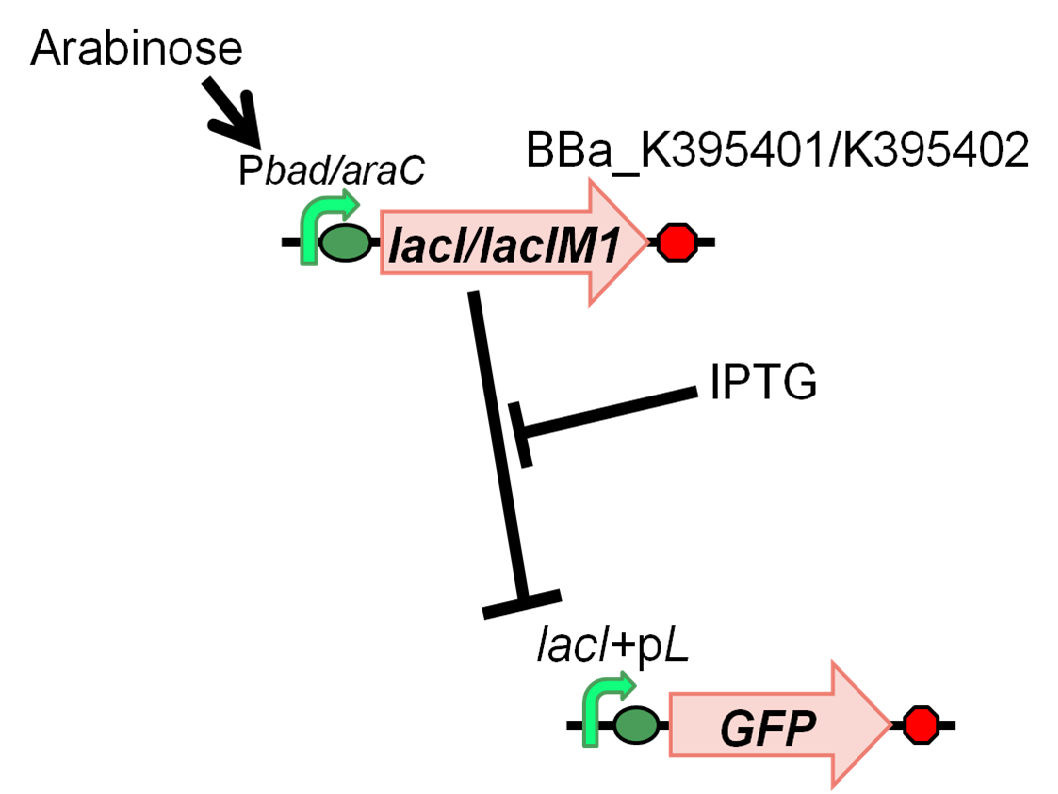
We characterized LacIM1 (BBa_K082026), a mutant of LacIWT, which is a key component in the band-detect network. This part was registered by USTC(2008) [2]. However, the sequence data of BBa_K082026 is incorrect and it was not well characterized in the BioBrick registry. Therefore, we registered this part as BBa_K395400 and confirmed that LacIM1 shows weaker repression to lac promoter than LacIWT. In order to measure the function of LacI proteins, we constructed following two plasmids, BBa_K395401 (LacIM1) and BBa_K395402 (LacIWT), which have an arabinose inducible promoter. We measured GFP expression dependent on the input of arabinose and IPTG (Fig. 4-2-1).
Introduction
What is LacIM1?
As I wrote before, LacIM1 shows weaker repression compared to LacIWT because of its lower affinity to lac promoter [1].
Requirement for band-detect network
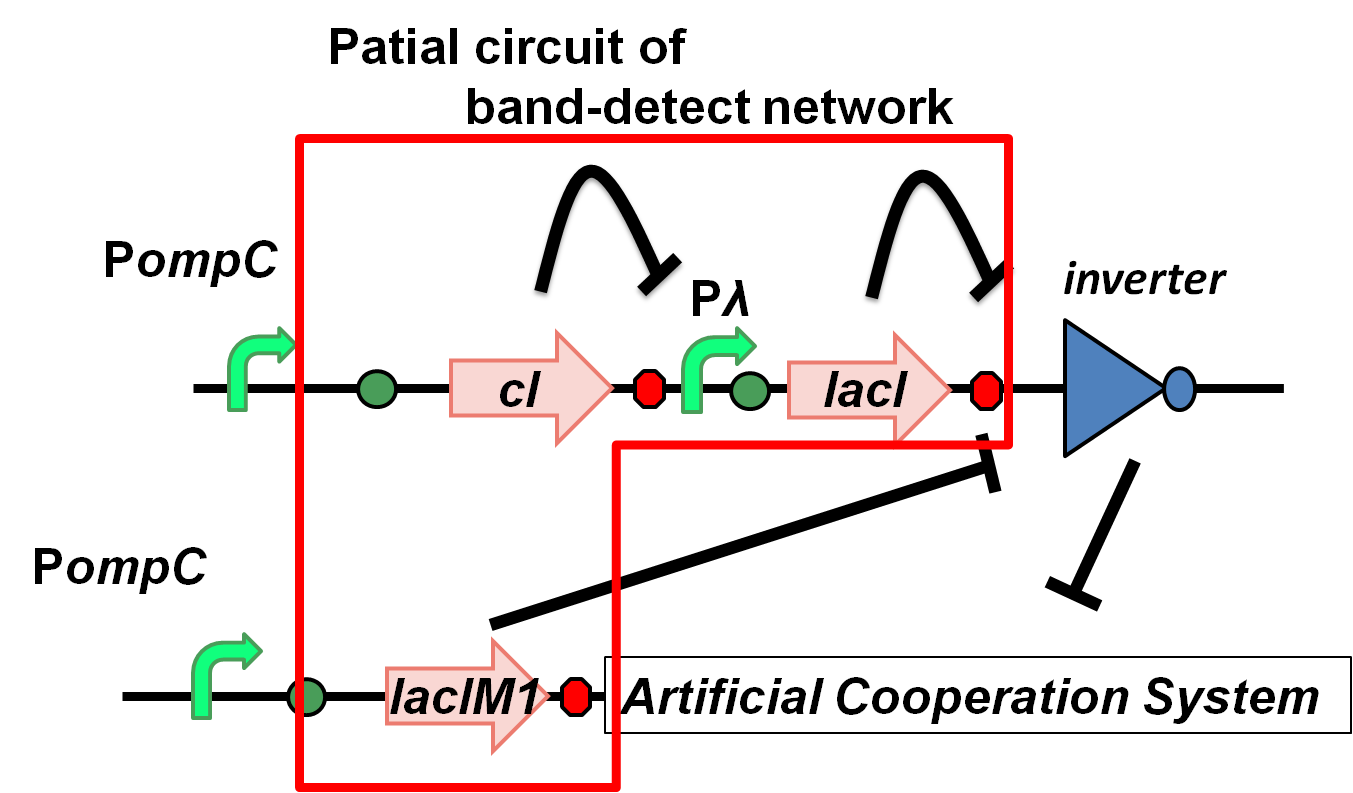
The band-detect network exhibits transient gene expression in response to concentration of chemical signals (Fig. 4-2-3). In the band-detect network, LacIM1 is a crucial component due to its low repression efficiency [1].
Results
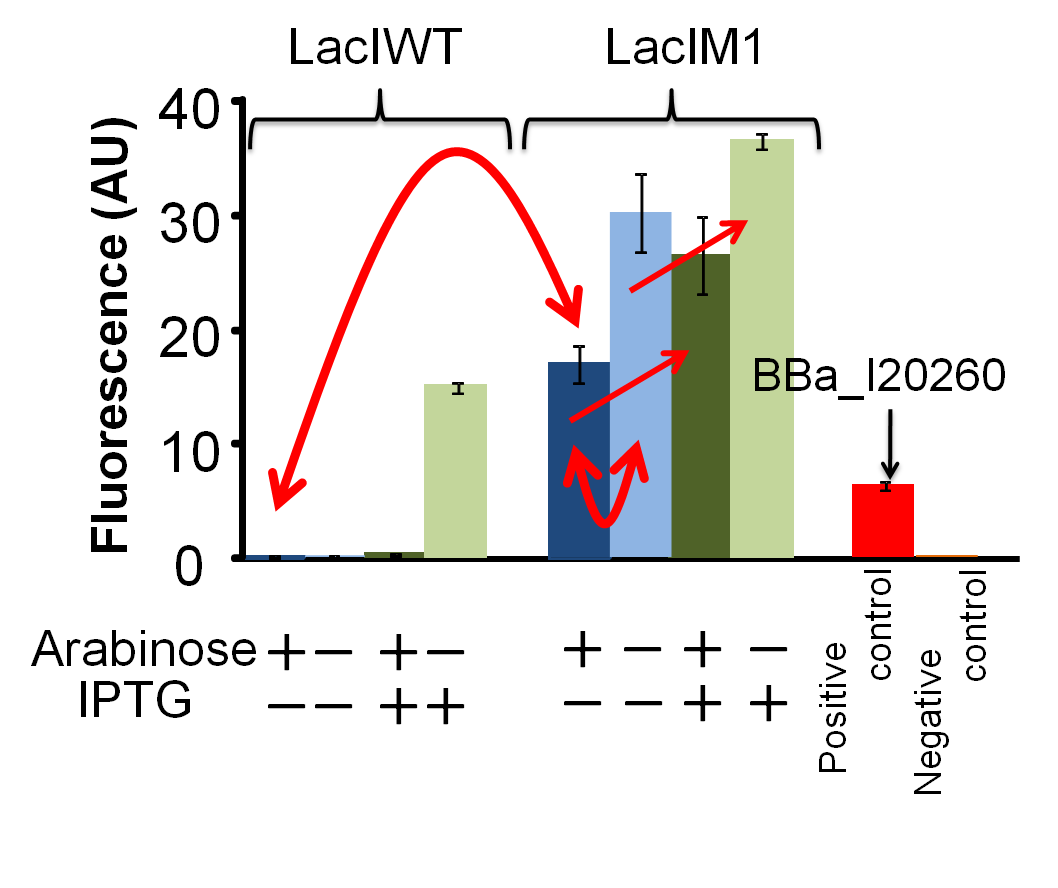
GFP expression is dependent on the input of arabinose and IPTG. After 4 hours of arabinose and IPTG induction, fluorescence intensities of the cultures were measured by fluorometer (FLA5000,FUJI).
As positive control, we used BBa_I20260, which is the measurement kit of the BioBricks.
Dark-blue bars, 0.2% arabinose and 0 mM IPTG;
blue bars, 0% arabinose and 0 mM IPTG;
dark-green bars, 0.2% arabinose and 1 mM IPTG;
green bars, 0% arabinose and 1 mM IPTG;
red bar, positive control
orange bar, negative control
(Fig4-2-2)
After 4 hours of arabinose and IPTG induction, the fluorescence intensities of the cultures were measured by fluorometer (FLA5000, FUJI).
LacIM1 shows weaker repression to lac promoter than LacIWT
We confirmed that LacIM1 (BBa_K082026) represses lac promoter but the repression is weaker than that by LacIWT (BBa_I732820). Figure. 4-2-2 shows that in the presence of arabinose and the absence of IPTG, the fluorescence intensity of the cell with LacIM1 was higher than that of the cell with LacIWT. This result shows that the repression by LacIM1 to lac promoter is weaker than that by LacIWT. In the absence of both arabinose and IPTG, the fluorescence intensity of the cell with LacIM1 was about 1.8-fold higher than that in the presence of arabinose and the absence of IPTG. From this result, we concluded that the GFP expression was repressed dependent on the LacIM1 expression induced by arabinose. In other words, the fact that LacIM1 represses the GFP expression was confirmed. Moreover, increase of GFP expression by addition of IPTG, with or without arabinose induction, also shows that the repressions are dependent on LacIM1.
Leaky expression of LacI proteins from Pbad promoter
We confirmed that leaky expressions of LacIM1 and LacIWT occurred respectively in the absence of arabinose. The fluorescence intensity of the culture with LacIM1 in the absence of both arabinose and IPTG was lower than that in the absence of arabinose and presence of IPTG. Without arabinose, the repression of GFP expression should not occur because the expression of LacIM1 does not occur. This result shows that the leaky expressed LacIM1 repressed GFP expression in the absence of IPTG. This leaky expression could also explain the results for LacIWT.
Overexpression of LacIWT inhibits the expression of GFP even in the presence of IPTG induction
We confirmed that the overproduction of LacIWT with arabinose induction inhibits the GFP expression even in the presence of IPTG. Although arabinose induces the overproduction of LacI protein that represses the GFP expression, the repression by LacI protein can be inhibited with IPTG induction. Therefore, we expected the occurrence of GFP expression in the presence of both arabinose and IPTG. However, there is no expression of GFP in the presence of both arabinose and IPTG. This result indicates that the amount of IPTG was not sufficient to inhibit the repression by overproduced LacIWT. In contrast, when there is only leaky expression of LacI protein without arabinose induction, addition of IPTG could fully inhibit the repression by LacIWT and the GFP expression occured. From this result, we concluded that the overexpression of LacIWT inhibited the expression of GFP even in the presence of IPTG induction.
Conclusion
From the results, we confirmed that LacIM1 shows weaker repression comparing to LacIWT. We characterized LacIM1(BBa_K082026/BBa_K395400), which is key part of building the “Wolf coli” system.
Materials&Methods
Construction of E.coli strain DH5α
In order to characterize LacIM1(K082026/K395400), LacI Mutant, we constructed BBa_K395401 combining I0500 and K082026(K395400) on pSB1A3 and used BBa_I20260 as a positive control and used promoterless ‘’gfp’’ on pSB3K3 as a negative control. Furthermore, we constructed BBa_K395402 combining I0500 and BBa_I732820 on pSB1A3 as a control experiment. As a GFP reporter, we used BBa_I7106 on pSB3K3. In order to measure the function of LacI proteins, we introduced BBa_I7106 (lacI+pL-rbs-GFP-ter) on pSB3K3 and BBa_K395401/BBa_K395402 into DH5α.
Report assay
Overnight cultures of reporter strains grown at 37 °C in LB Medium containing appropriated antibiotics were diluted at least 1:100 in the medium and were incubated at 37 °C as fresh cultures. After their OD590 reached 0.3, added appropriate inducers that 1mM IPTG, and/or 0.2% arabinose (final concentration). After 4 hours of induction, 150 μl of each sample was applied to 96-well plate and its fluorescence intensity was measured with a fluorometer by BPB-sp filter, which absorbed the LB fluorescence. The fluorescence intensity was calculated by dividing the measured raw fluorescence intensity by the adjusted OD590.
Reference
[1]. Basu S et al. A synthetic multicellular system for programmed pattern formation. NATURE 2005;434,1130-1134
[2]. USTC(2008)
 "
"