Team:Newcastle/27 August 2010
From 2010.igem.org

| |||||||||||||
| |||||||||||||
Contents |
PCR and digestion controls
Aims
Since we have recently had problems with both PCR and EcoR1 restriction digests, we set up positive controls to ensure that there is nothing wrong with our enzymes or buffers. We performed a single digest of pGFP-rrnb with EcoR1 and PCR of pSB1C3 and a fragment of the rocF coding sequence from Bacillus subtilis 168 genomic DNA.
Materials and Protocols
Please refer to the restriction digest, Phusion PCR and gel electrophoresis protocols.
Results
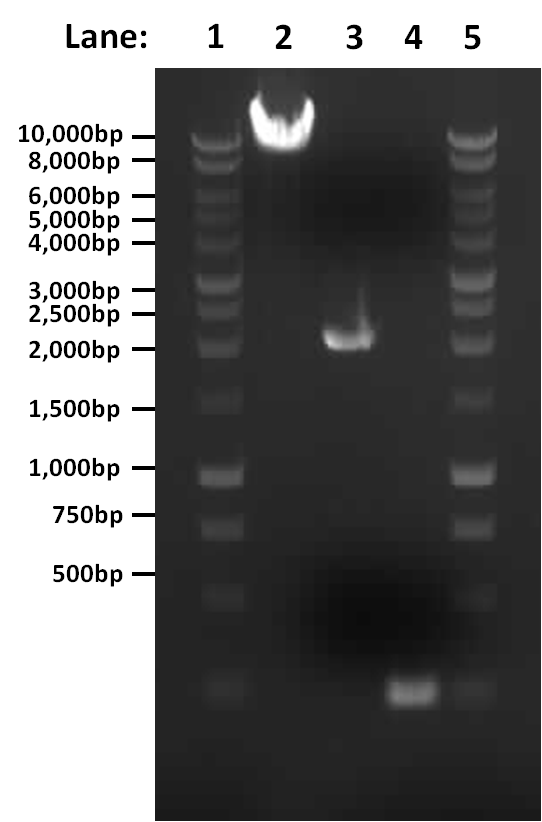
Figure 1: Gel electrophoresis of the amplified PCR products and restriction digest
- Lane 1: 1 Kb ladder
- Lane 2: digested (linearised) pGFP-rrnB
- Lane 3: PCR product of pSB1C3
- Lane 4: PCR product of first fragment of rocF coding sequence
- Lane 5: 1 Kb ladder
Discussion
The single digest and two PCR reactions both worked correctly.
yneA
Starch plating
Aims
Yesterday we replica plated Bacillus subtilis 168, that were transformed on Wednesday, onto starch plates. Today we aim to see whether or not the transformed Bacillus subtilis can breakdown starch. If they cannot break down starch there will be no halo when we test with iodine.
Materials
- Starch plates
- Pipette tips
- Iodine
Results
Unfortunately our colonies had halos. Although the insert has integrated, the colonies have antibiotic restistance, the insert has not integrated at the amyE locus.
yneA
PCR (Repeat)
Aim
To repeat the PCR that we did yesterday using the correct rocF primers.
Materials and Protocol
Please refer to PCR.
PCR Purification
Aim
To remove unwanted primers, taq polymerase, buffer and salts to obtain pure DNA.
Materials and Protocol
Please refer to PCR purification.
Digestion
Aim
To digest the PCR products of pSB1C3 and yneA from PCR purification.
Materials and Protocol
Please refer to restriction digest.
Results, Discussion and Conclusion
We run the digested products with gel electrophoresis to determine whether the digest worked.
Gel extraction
Aim
To purify the DNA of yneA and pSB1C3 by extracting the bands from the gel after running gel electrophoresis. Concentration of DNA is then checked with NanoDrop.
Materials and Protocol
Please refer to:
Results
When we look at the gel through the GelDoc, only pSB1C3 was successfully digested with EcoR1 and Pst1 while yneA did not show any bands.
Conclusion
We will repeat the protocol for yneA on 31.08.10.
 
|
 "
"