Team:Newcastle/Gel extraction
From 2010.igem.org

| |||||||||||||
| |||||||||||||
QIAquick Gel Extraction Microcentrifuge Protocol
Materials
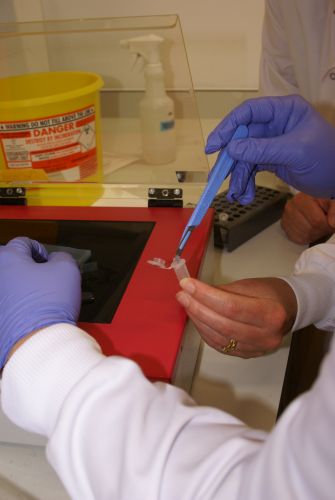
- Scalpel
- Eppendorf tubes
- Pipettes
- QIAquick column
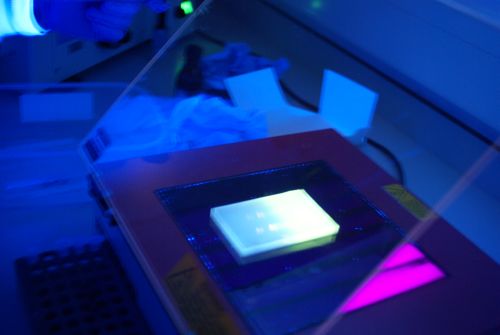
- UV transluminator
- Buffer QG
- Buffer PE
- Buffer EB
- Isopropanol
- 70% ethanol
Protocol
- Before extraction, clean the UV transilluminator and scalpel with 70% ethanol.
- Excise the DNA fragment from the agarose gel with a clean, sharp scalpel. ( Minimise the exposure of the gel to UV as much as possible.)
- Weight the gel slice and add 3 volumes of buffer QG to 1 volume of gel (100 mg ~ 100 µl).
- Incubate at 50°C and invert the tube gently at regular interval until the gel has completely dissolved.
- After the gel has dissolved completely, check that the color of the mixture is yellow.
- Add 1 gel volume of isopropanol to the sample and mix.
- Place a QIAquick spin column in a 2 ml collection tube.
- To bind DNA, apply the sample to the QIAquick column and centrifuge for 1 min.
- Discard the flow through and place the QIAquick column back into the same tube (max volume: 750 µl).
- Add 0.5 ml of Buffer QG to QIAquick column and centrifuge for 1 min. Discard the flow through and place the QIAquick column back into the same tube.
- To wash, add 0.75 ml of Buffer PE to QIAquick column and centrifuge for 1 min. Place the QIAquick column back into the same tube.
- Centrifuge the column for a further 1 min.
- Transfer the column into a clean 1.5 ml microcentrifuge tube.
- To elute DNA, add 30 µl of Buffer EB to the center of the QIAquick membrane and allow to stand for 1 min.
- Centrifuge the column for 1 min and transfer the eluate to a clean 1.5 ml micriocentrifuge tube.
- To measure the purity of the sample, use a Nanodrop Spectrophotometer.
Go back to our Protocol List
 
|
 "
"