Team:Tokyo Tech/Project/Apple Reporter
From 2010.igem.org
Contents |
Apple Reporter
Color
overview
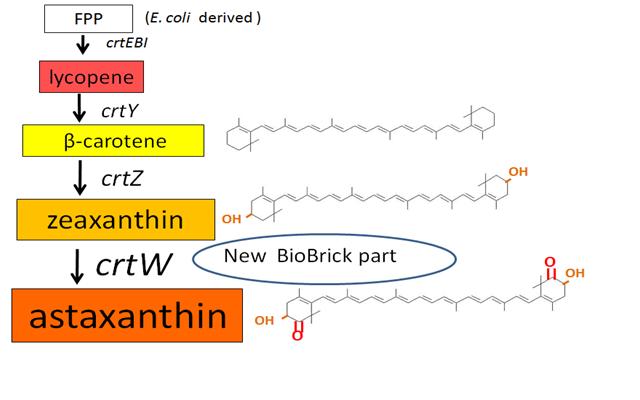
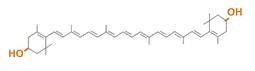
Carotenoids are natural organic pigments in plants and bacteria. Synthetic carotenoid pigments colored yellow, red or orange represent about 15-25% of the cost of production of commercial feed. Carotenoids are terpenoid based on a structure having the chemical formula C40H56. There are about 600 carotenoids, which perform a range of functions. For example, light energy absorption, protection against photo-damage, acting as antioxidants, and as precursor to other organic compounds such as vitaminA. Production of pigment has been worked in iGEM, and quite a few parts for synthesis of carotenoid-related compounds already exist in BioBricks Registry. Since the carotenoid synthetic pathway is huge, BioBricks Registry doesn’t cover all parts to complete the pathway. This time, we intorduced some new BioBrick parts to synthesize more various kinds of carotenoids. And we succeeded in synthesizing astaxanthin, one of the final metabolite of the pathway. Also, astaxanthin is not converted to vitaminA in the human body. Too much vitamin A is toxic for a human, but astaxanthin has lower toxicity. Furthermore, as the synthetic pathway is completed, it becomes possible to change the hue gradually by controlling the production of several carotenoids.
~carotenoid synthetic pathway~
works
・ β-carotene synthesis
→more information about β-carotene
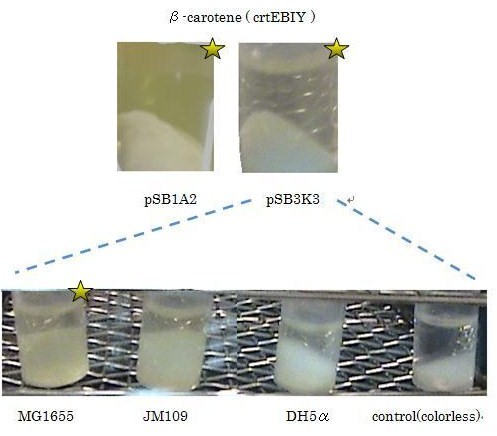
Our team synthesized β-carotene under several conditions to confirm the activity of BioBrick parts designed by Cambredge 2009.
We used crtEBIY (BBa_K274210) to synthesize β-carotene. We compared the plasmids containing pSB3K3 (low-copy vector) with the plasmids containing pSB1A2 (high-copy vector) from the production. We also introduced the low-copy plasmid into E. Coli strain MG1655, JM109 and DH5α respectively.
~acetone extract~
→protocol
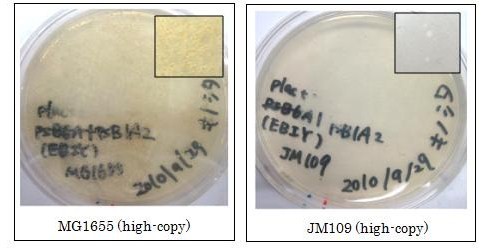
We found that MG1655 and JM109 produce more β-carotene than DH5α in liquid culture and that MG1655 grow better than JM109 on plate culture. These results indicated that MG1655 is the best of the three strains to treat. For this reason, we decided to use only MG1655 to extract pigment.
We also made special competent cell, MG1655 and JM109, having a plasmid (crtEBIY; pSB1A2 or crtEBIY; pSB3K3) for zeaxanthin and astaxanthin synthesis.(→figure)
・ zeaxanthin synthesis
→more information about zeaxanthin
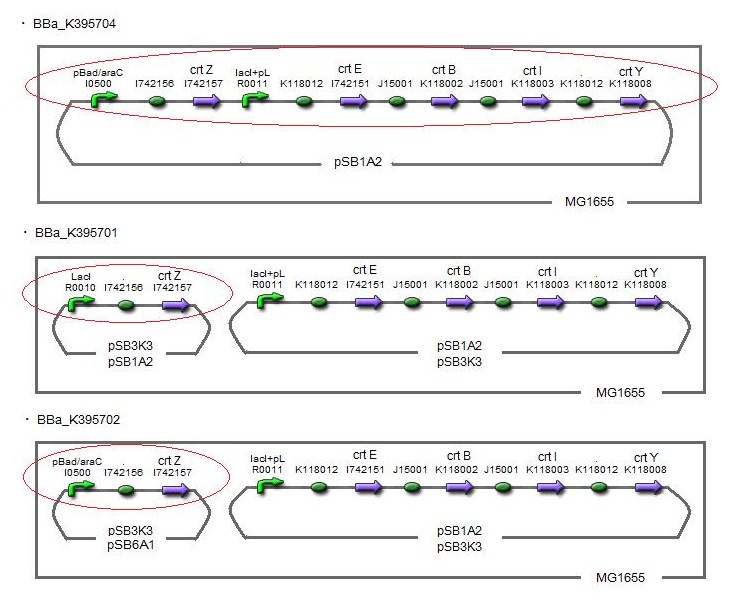
Our team synthesized zeaxanthin by several kinds of construct to confirm the activity of BioBrick parts designed by Cambredge 2009 and Edinburgh 2007. By assembling the crtZ, crtEBIY and appropriate promoters, we constructed new BioBrick parts to synthesize zeaxanthin and compared their function . (→BBa_K395701, BBa_K395702, BBa_K395704)
We constructed two types of zeaxanthin synthetic construct.
One is that crtZ (BBa_I742158) and crtEBIY (BBa_K274210) were assembled on a single plasmid.(We call this construct “single plasmid construct”.)
The other is that crtZ and crtEBIY were assembled on different plasmids.(we call this type of constructs “double plasmids construct”.)
We made 5 combinations shown in table<1>.
Fragrance
overview
Apple fragrance is featured by combinations of volatile compounds, including alcohols, aldehydes, ketones, sesquiterpenes and esters. Esters are associated with fruity attributes of fruit fragrance. In the commercial apple cultivar, over 30 esters have been identified.[Ref]The last step in ester biosynthesis is catalyzed by alcohol acyl transferases (AATs) that use coenzyme A(CoA) donors together with alcohol acceptors as substrates. We learned it’s able to make E. coli produce apple fragrance by adding the gene MpAAT1 because Acetyl CoA exists originally in E.coli. MpAAT1 produces a predicted protein containing character of other plant acyl transferases. In contrast with production of pigment, production of fragrance has hardly been worked in the iGEM and few parts exist.For these reasons, we introduced a new BioBrick part to synthesize components of apple fragrance.
Highlight
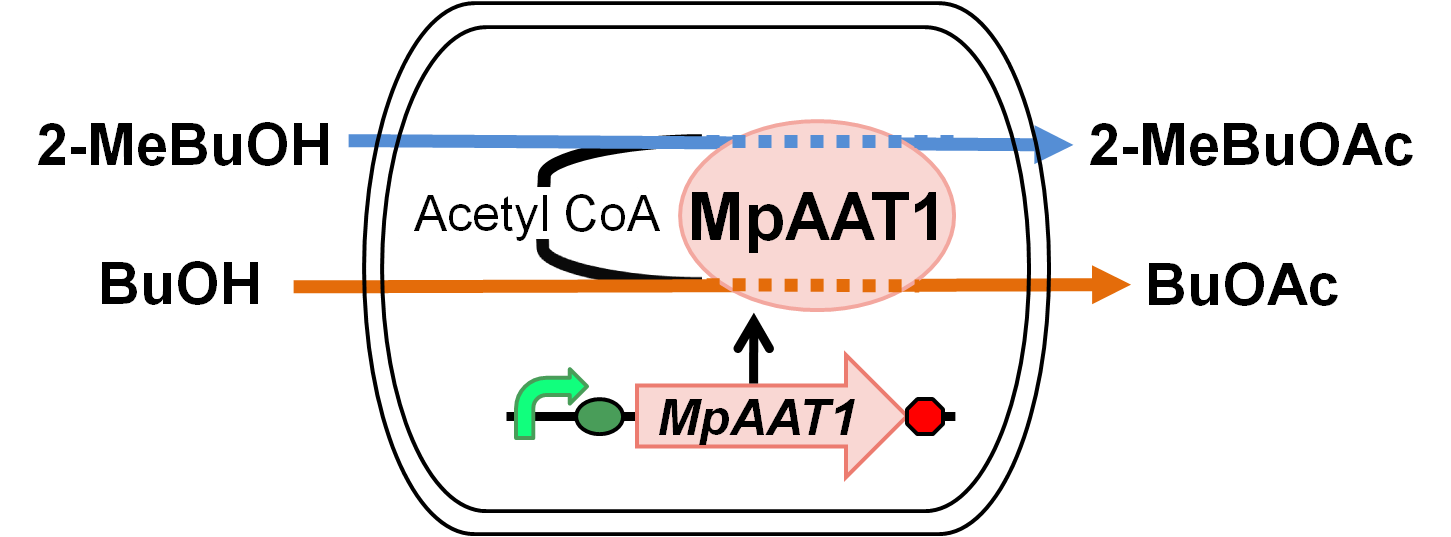
We designed apple fragrance expression system with MpAAT1. Fig. 1.2.1 shows the outline of the system. MpAAT1 converts substrate and Acetyl CoA into apple fragrance. Acetyl CoA exists originally in E.coli cell.When 2-methyl butanol or butanol is added as substrates, 2-methylbutyl acetate or butyl acetate is produced and it gives off apple fragrance.
We performed gas chromatography to confirm the production of the esters, and the results revealed that MpAAT1 successfully converted alcohol and Acetyl CoA into our target esters.
Result
We transformed MpAAT1(BBa_K395602) on pSB6 along with pTrx6 into E.coli BL21 DE3, and cultured after addition of alcohols(2-MeBuOH or BuOH) as substrates. After 12 hours of incubation, we extracted organic soution layer from the culture and analyzed by gas chromatography (Fig. 1.2.2). Peaks of the esters producing apple fragrance(2-MeBuOAc or BuOAc) were detected.
We designed and constructed a system for apple fragrance production from intracellular metabolites (BBa_K395602). We analyzed cultures with the apple fragrance biosynthetic system for Butyl acetate and 2-methylbutyl acetate production and measured by gas chromatography.(A)Apple fragrance generator (BBa_K395602) in the presence of butanol produces measurable quantities of butyl acetate.(B)Apple fragrance generator (BBa_K395602) does not produce measurable quantities of butyl acetate in the absence of butanol.(C)For reference, we analyzed pure butyl acetate.(D)Apple fragrance generator (BBa_K395602) in the presence of 2-methyl butanol produces measurable quantities of 2-methylbutyl acetate.(E)Apple fragrance generator (BBa_K395602) in the absence of 2-methyl butanol does not produce measurable quantities of 2-methylbutyl acetate(F)For reference, we analyzed pure 2-metylbutyl acetate.(G)For negative control, we analyzed with no sample. Undecane was used as an internal standard for all samples.
Discussion
From the result of the experiment above, we can conclude that engineered E.coli successfully converted alcohols added as substrates into esters. Moreover, we synthesized MpAAT1 and esters using E.coli BL21 DE3 as a chassis, which was reported to be implausible in former report(s) (C.R. Shen, J.C. Liao. 2008). In 2008, C.R. Shen and J.C. Liao succeeded in synthesizing butanol from E.coli. If we take advantage of this engineered E.coli, we could produce apple fragrance ester without the addition of substrate.
 "
"