Team:Tokyo Tech/Project/Apple Reporter
From 2010.igem.org
(→zeaxanthin synthesis) |
|||
| Line 100: | Line 100: | ||
| - | [[Image:tokyotech_Fig. 1.1.7.1.BBa_K395704.jpg|left| | + | [[Image:tokyotech_Fig. 1.1.7.1.BBa_K395704.jpg|left|500px|thumb|S1]] |
| - | [[Image: | + | [[Image:TLC2.png|left|300px|thumb|worked by Yumiko Kinoshita]] |
| + | [[Image:Tokyotech_zeaxanthin_synthesis.jpg|center|500px|thumb|worked by Yumiko Kinoshita]] | ||
| - | |||
| - | |||
| - | |||
[[Image:tokyotech_Fig. 2-1-14-3TLC zeaxanthin.png|left|150px|thumb|worked by Yumiko Kinoshita]] | [[Image:tokyotech_Fig. 2-1-14-3TLC zeaxanthin.png|left|150px|thumb|worked by Yumiko Kinoshita]] | ||
Revision as of 19:13, 27 October 2010
2-1 Color
Contents |
Abstract
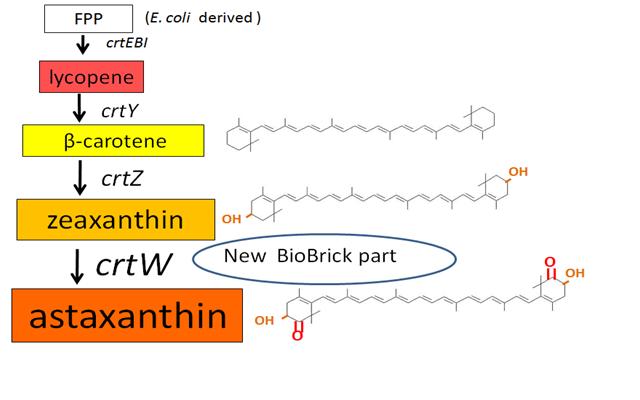
Our team synthesized β-carotene, zeaxanthin and astaxanthin under several conditions (strain, construct,…etc) to confirm the functions. Particularly, astaxanthin is the final metabolite of the carotenoid synthetic pathway.
We synthesized β-carotene and zeaxanthin by assembling crtEBIY and crtZ of existing BioBrick parts. Though the part crtZ has been unconfirmed, we confirmed it for the first time in iGEM.
In addition, we succeeded in synthesizing astaxanthin by introducing the new BioBrick part crtW. Therefore, the carotenoid synthetic pathway reached the end.
Thus, we performed Thin Layer Chromatography (TLC) to confirm the products of β-carotene, zeaxanthin, astaxanthin were synthesized.
~carotenoid synthetic pathway~
Introduction
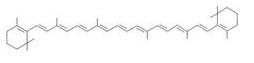
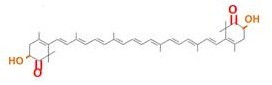
Carotenoids are natural organic pigments in plants and bacteria. Synthetic carotenoid pigments colored yellow, red or orange represent about 15-25% of the cost of production of commercial feed. Carotenoids are terpenoid based on a structure having the chemical formula C40H56. There are about 600 carotenoids, which perform a wide range of functions. (For example, light energy absorption, protection against photo-damage, acting as antioxidants, and as precursors to other organic compounds.) Particularly, astaxanthin is not converted to vitamin A in the human body. Too much vitamin A is toxic for a human, but astaxanthin has lower toxicity.
Previous year, our team has completed melanin synthetic pathway. Since the carotenoid synthetic pathway is huge, BioBrick Registry doesn’t cover all parts to complete the pathway. This year, we introduced a new BioBrick part to synthesize more various kinds of carotenoids. And we succeeded in synthesizing astaxanthin, one of the final metabolite of the pathway.
Furthermore, when the synthetic pathway is completed, it becomes able to change the color gradually by controlling the production of several carotenoids.
Results
β-carotene synthesis
Our team synthesized β-carotene under several conditions to confirm the functions of BioBrick parts designed by Cambridge 2009. (See more...)
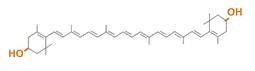
zeaxanthin synthesis
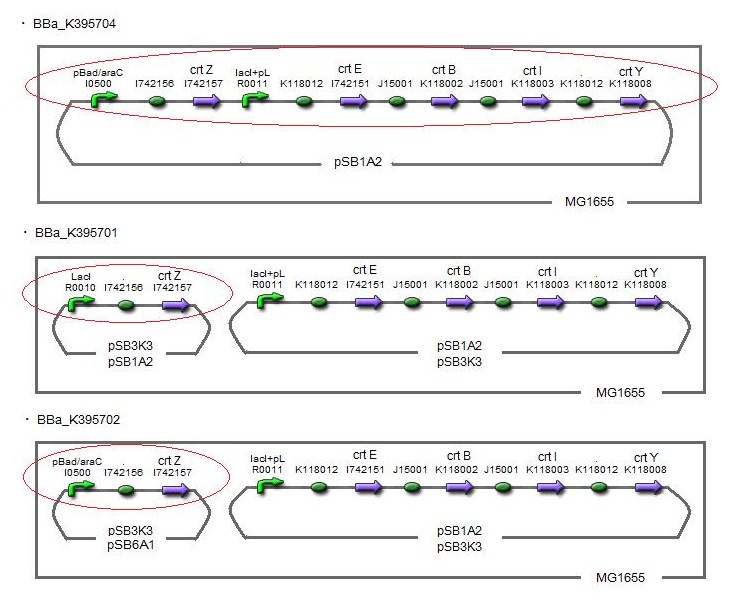
Our team synthesized zeaxanthin by several kinds of construct to confirm the functions of BioBrick parts designed by Cambridge 2009 and Edinburgh 2007. By assembling the crtZ, crtEBIY and appropriate promoters, we constructed new BioBrick parts to synthesize zeaxanthin. Then we separated the products of zeaxanthin by TLC. Especially, we confirmed the part crtZ for the first time in iGEM. (→[http://partsregistry.org/wiki/index.php?title=Part:BBa_K395701 BBa_K395701], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K395702 BBa_K395702], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K395704 BBa_K395704])
We constructed two types of zeaxanthin synthetic construct.
One is that crtZ(BBa_I742158) and crtEBIY(BBa_K274210) were assembled on a single plasmid.(We call this construct “single plasmid construct”.) The other is that crtZ and crtEBIY were assembled on different plasmids.(we call this type of constructs “double plasmids construct”.)
We made 5 combinations shown in table<1>.
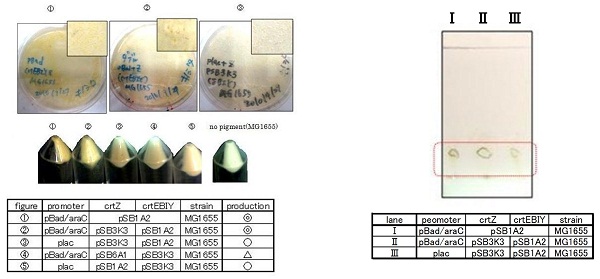
Constructs containing pBad/araC promoter on high-copy vector show the best production and double plasmids construct shows as much production as single plasmid construct under same promoter combination. Construct with pBad/araC promoter shows better production than that with plac promoter. Construct that crtEBIY on high-copy vector and crtZ on low-copy vector shows as much expression as construct that crtEBIY on low-copy vector and crtZ on high-copy vector.(Actually, #3 showed a little more production than #5.)
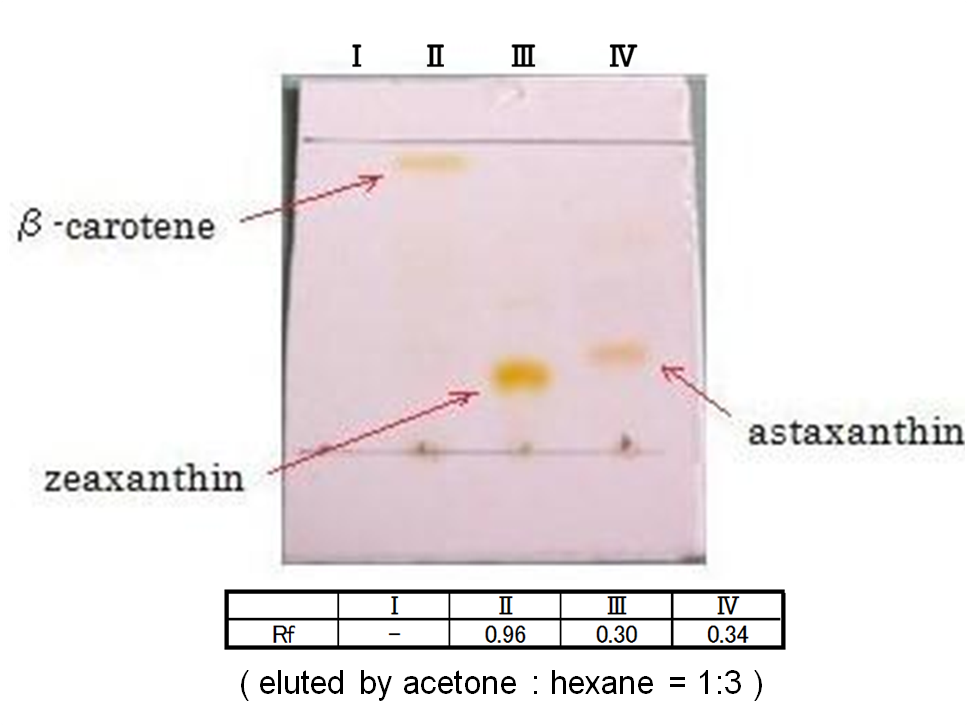
We selected the best three samples for TLC. In the consequence of TLC, we could confirm that these three samples were zeaxanthin.
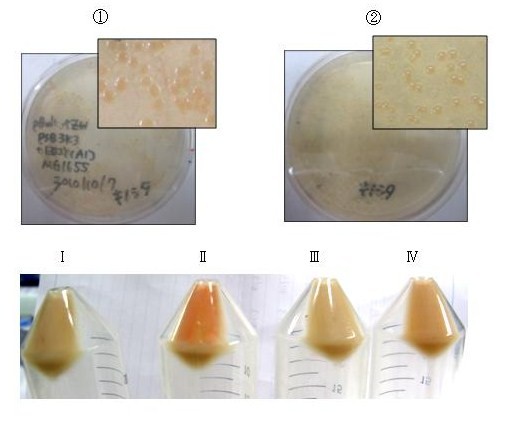
astaxanthin synthesis
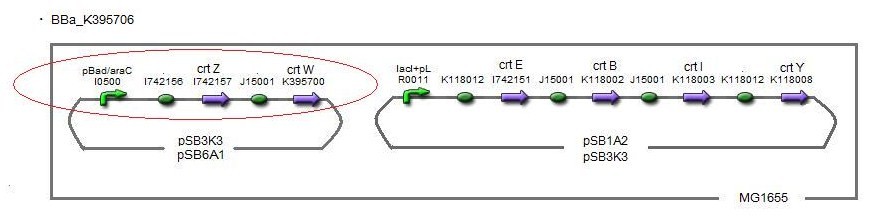
Our team synthesized astaxanthin for the first time in iGEM. Thus the carotenoid synthetic pathway reached the end. This work was done by assembling the new BioBrick part crtW and zeaxanthin synthesis constructs we designed. From the result of “zeaxanthin synthesis”, we decided to use pBad/araC promoter and double plasmids construct. After constructing a new BioBrick device ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K395706 BBa_K395706]) to synthesize astaxanthin, we examined the functions under four conditions shown in table<2>. We finally could confirm the products of astaxanthin by TLC.
The activity of pBad/araC promoter is measured in detail on another page. →click
~acetone extract and vacuum concentration~ →protocol
Not only astaxanthin but also another pigments were priduced at Ⅱ.
We regarded them as intermediary metabolites between zeaxanthin and astaxanthin.
Reference
[1]N.M. Sachindra, et al. Carotenoids in crabs from marine and fresh waters of India. LWT 38 (2005) 221–225
[2]MISAWA N, et al. Structure and Functional Analysis of a Marine Bacterial Carotenoid Biosynthesis Gene Cluster and Astaxanthin Biosynthetic Pathway Proposed at the Gene Level. JOURNAL OF BACTERIOLOGY, Nov. 1995, p. 6575–6584 Vol. 177, No. 22
[3]Nishizaki T, et al. Metabolic engineering of carotenoid biosynthesis in Escherichia coli by ordered gene assembly in Bacillus subtilis. Appl Environ Microbiol. 2007 Feb
[4]Seon-Kang C, et al. Characterization of bacterial β-carotene 3,3′-hydroxylases, CrtZ, and P450 in astaxanthin biosynthetic pathway and adonirubin production by gene combination in Escherichia coli. Appl Microbiol Biotechnol (2006) 72
[5]Arunkumar K, et al. Structure of a carotenoid gene cluster from Pantoea sp. strain C1B1Y and characterization of β-carotene hydroxylase(crtZ)gene by functional complementation in Escherichia coli. Carotenoid Science Vol.11 (2007)
appendix
β-carotene synthesis
Our team synthesized β-carotene under several conditions to confirm the activity of BioBrick parts designed by Cambredge 2009.
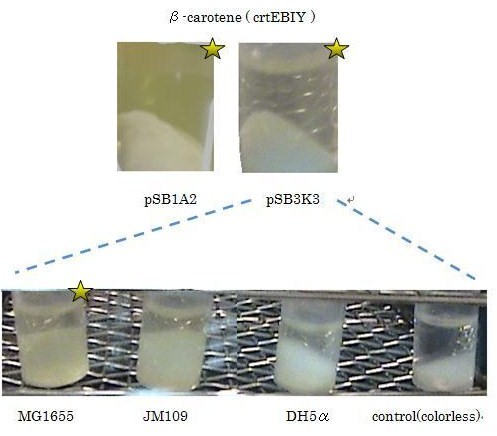
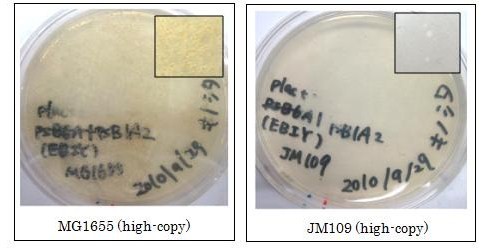
We used crtEBIY (BBa_K274210) to synthesize β-carotene. We compared the plasmids containing pSB3K3 (low-copy vector) with the plasmids containing pSB1A2 (high-copy vector) from the production. We also introduced the low-copy plasmid into E. Coli strain MG1655, JM109 and DH5&alfa; respectively.
We found that MG1655 and JM109 produce more β-carotene than DH5α in liquid culture and that MG1655 grow better than JM109 on plate culture. These results indicated that MG1655 is the best of the three strains to treat. For this reason, we decided to use only MG1655 to extract pigment.
We also made special competent cell, MG1655 and JM109, having a plasmid crtEBIY; pSB1A2 or crtEBIY; pSB3K3) for zeaxanthin and astaxanthin synthesis.(→S1, S2)
~acetone extract~
→protocol
Materials and Methods
The work flow to TLC from culture
1. The cells were cultured over night.
2. 50ul bacterial sution was taken from culture 1 and moved into 50ml LB culture with appropriate antibiotic. Inducer was added,if necessary.
3. The cells were incubated at 37℃24hour.
4. After the O.D reach 2.0, culture was centrifuged for 10minutes at 4℃, 6000rpm,10min
5. Supernatant was removed by decanting. The remainder was added with 1 ml ddH20 and vortexed.
6. Moved into 2ml tube by using P1000 pipetter at least twice.
7. Solution is centrifuged for 20minutes at 4℃, 14000rpm
8. Supernatant was removed completely using pipetter. About 100ul of pellet is obtained.
9. Pellet is added with 500ul acetone, and vortexed for 10 minutes to extract the pigment.
10. Solution is centrifuged for 20minutes at 4℃, 14000rpm
11. Acetone solution was replaced into 2 ml tube.
12. Acetone was removed using rotary evaporator.
13. 50 ul of Chloroform/Methanol solution(weight ratio of 9:1) was added.
14. Sample was vortexed and spinned down.
15. The water layer was removed.
16. Spot the sample onto TLC silica gel plate, and developed by acetone/hexane solution.(weight ratio of 1:3)
17. Spot was observed under visible light. Tokyotech_zeaxanthin_synthesis.jpg Tokyotech_astaxanthin_synthesis.jpg
 "
"