Team:Tokyo Tech/Project/Apple Reporter
From 2010.igem.org
(→Hilight) |
|||
| Line 45: | Line 45: | ||
| - | == | + | ==Highlight== |
==Works== | ==Works== | ||
Revision as of 13:53, 26 October 2010
Contents |
Color
Introduction
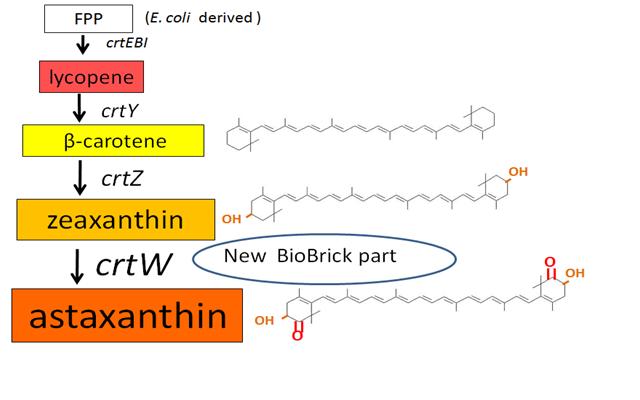
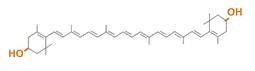
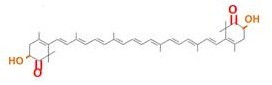
Carotenoids are natural organic pigments in plants and bacteria. Synthetic carotenoid pigments colored yellow, red or orange represent about 15-25% of the cost of production of commercial feed. Carotenoids are terpenoid based on a structure having the chemical formula C40H56. There are about 600 carotenoids, which perform a range of functions. For example, light energy absorption, protection against photo-damage, acting as antioxidants, and as precursor to other organic compounds such as vitaminA. However, astaxanthin is not converted to vitaminA in the human body. Too much vitamin A is toxic for a human, but astaxanthin has lower toxicity. Works of synthesizing pigment have been done in iGEM, and our team has completed melanin synthetic pathway last year. Since the carotenoid synthetic pathway is huge, BioBricks Registry doesn’t cover all parts to complete the pathway. This year, we intorduced a new BioBrick part to synthesize more various kinds of carotenoids. And we succeeded in synthesizing astaxanthin, one of the final metabolite of the pathway. Furthermore, when the synthetic pathway is completed, it becomes able to change the color gradually by controlling the production of several carotenoids.
~carotenoid synthetic pathway~
Highlight
Works
β-carotene synthesis
→more information about β-carotene
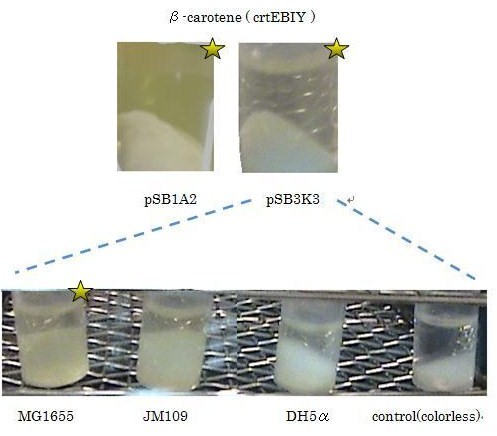
Our team synthesized β-carotene under several conditions to confirm the activity of BioBrick parts designed by Cambredge 2009.
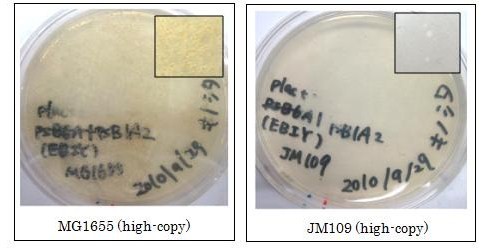
We used crtEBIY (BBa_K274210) to synthesize β-carotene. We compared the plasmids containing pSB3K3 (low-copy vector) with the plasmids containing pSB1A2 (high-copy vector) from the production. We also introduced the low-copy plasmid into E. Coli strain MG1655, JM109 and DH5α respectively.
~acetone extract~
→protocol
We found that MG1655 and JM109 produce more β-carotene than DH5α in liquid culture and that MG1655 grow better than JM109 on plate culture. These results indicated that MG1655 is the best of the three strains to treat. For this reason, we decided to use only MG1655 to extract pigment.
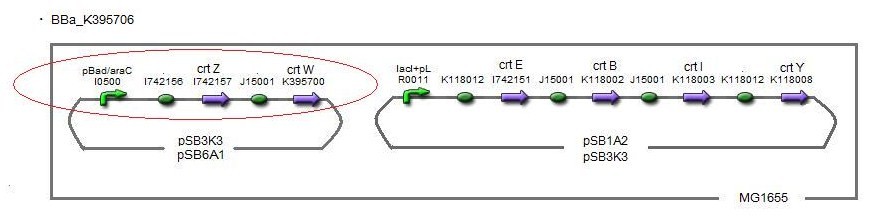
We also made special competent cell, MG1655 and JM109, having a plasmid crtEBIY; pSB1A2 or crtEBIY; pSB3K3) for zeaxanthin and astaxanthin synthesis.(→figure)
zeaxanthin synthesis
→more information about zeaxanthin
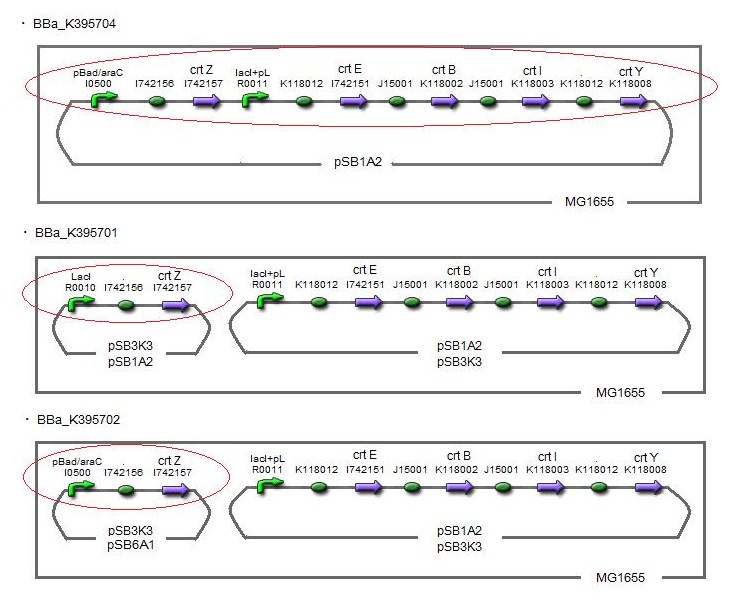
Our team synthesized zeaxanthin by several kinds of construct to confirm the activity of BioBrick parts designed by Cambredge 2009 and Edinburgh 2007. By assembling the ‘‘crtZ’’, ‘‘crtEBIY’’ and appropriate promoters, we constructed new BioBrick parts to synthesize zeaxanthin and compared their function . (→[http://partsregistry.org/wiki/index.php?title=Part:BBa_K395701 BBa_K395701], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K395702 BBa_K395702], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K395704 BBa_K395704])
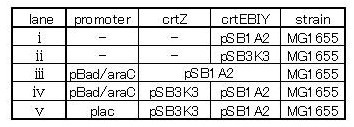
We constructed two types of zeaxanthin synthetic construct.
One is that crtZ(BBa_I742158) and crtEBIY(BBa_K274210) were assembled on a single plasmid.(We call this construct “single plasmid construct”.)
The other is that crtZ and crtEBIYwere assembled on different plasmids.(we call this type of constructs “double plasmids construct”.)
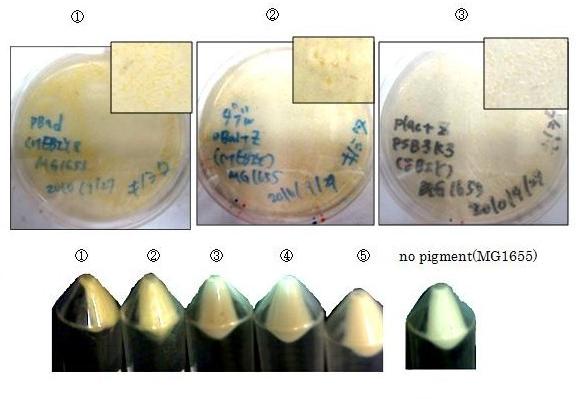
We made 5 combinations shown in table<1>.
Constructs containing pBad/araC promoter on high-copy vector show the best pruduction and double plasmids construct shows as much pruduction as single plasmid construct under same promoter conbination. Construct with pBad/araC promoter shows better production than that with plac promoter. Construct that crtEBIY on high-copy vector and crtZ on low-copy vector shows as much expression as construct that crtEBIY on low-copy vector and crtZ on high-copy vector.(Actually, ③ showed a little more production than ⑤.)
From the result, we decided to use pBad/araC promoter and double plasimds construct.
astaxanthin synthesis
→more information about astaxanthin
Our team synthesized astaxanthin to make the pathway reach the end by assembling crtW and zeaxanthin synthesis constructs. After constructing new BioBrick parts to synthesize astaxanthin we compared their function. (→[http://partsregistry.org/wiki/index.php?title=Part:BBa_K395706 BBa_K395706]), We also examine the production controlled by activity of pBad/araC promoter. The activity of this promoter is measured in detail on another page. →click
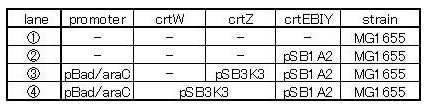
We made 4 combinations shown in table<2>
~acetone extract and vacuum concentration~ →protocol
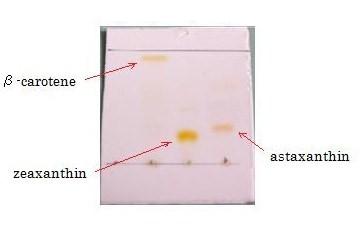
Separation of carotenoids with TLC
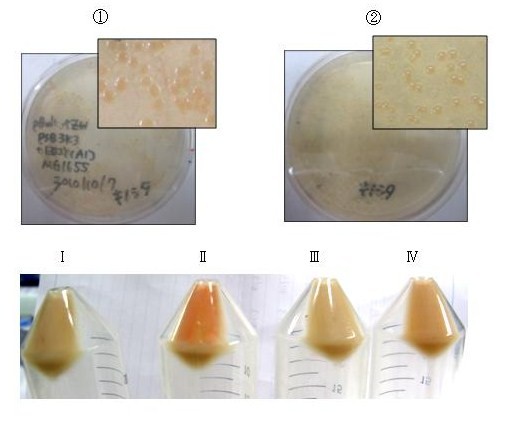
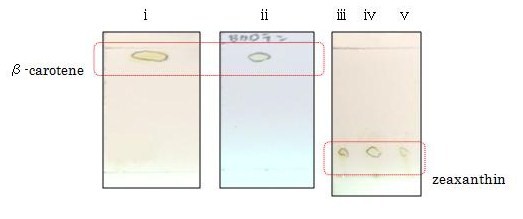
We performed thin layer chromatography (TLC) to confirm the products of β-carotene, zeaxanthin, astaxanthin were synthesized. (→protocol) The lanes are ①control(FPP), ②β-carotene, ③zeaxanthin, ④astaxanthin from the left. We can tell ②,③ and ④ are yellow-orange, golden-yellow and red-orange pigment, respectively. These results are consistent with the Rf and color of β-carotene, zeaxanthin, astaxanthin respectively and indicated that target products were synthesized as expected. [Reference: Carotenoids in crabs from marine and fresh waters of India ]
β-carotene and zeaxanthin
astaxanthin
Not only astaxanthin but also another pigments were priduced at Ⅱ;.
We regarded them as intermediary metabolites between zeaxanthin and astaxanthin.
 "
"