Team:Newcastle/13 August 2010
From 2010.igem.org
Shethharsh08 (Talk | contribs) (→rocF BioBrick gel electrophoresis) |
(→Gel electrophoresis of subtilin immunity BioBrick fragments) |
||
| (18 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Team:Newcastle/mainbanner}} | {{Team:Newcastle/mainbanner}} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | = | + | =Gel electrophoresis of subtilin immunity part fragments= |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Aims== | ==Aims== | ||
| - | |||
| - | + | The aim of this experiment is to do gel extraction for the ''spaIFEG'' gene cluster amplifed on the 12th August, 2010 and the Plasmid Vector, Promoter & RBS and Double terminator amplifed on the 11th August, 2010. Finally, NanoDrop will be performed for all the fragments. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Materials and protocol== | ==Materials and protocol== | ||
| - | Please refer to the | + | Please refer to the [[Team:Newcastle/Gel_extraction| gel extraction]] and [[TeamNewcastleNanoDrop_Spectrophotometer| NanoDrop]] protocols. Instead of using the centrifuge to bind the DNA from our sample to the QIAquick column, a '''vacuum manifold''' was used instead. It is the first time it had been used. The advantage of this was that the extraction could be carried out all at the same time. |
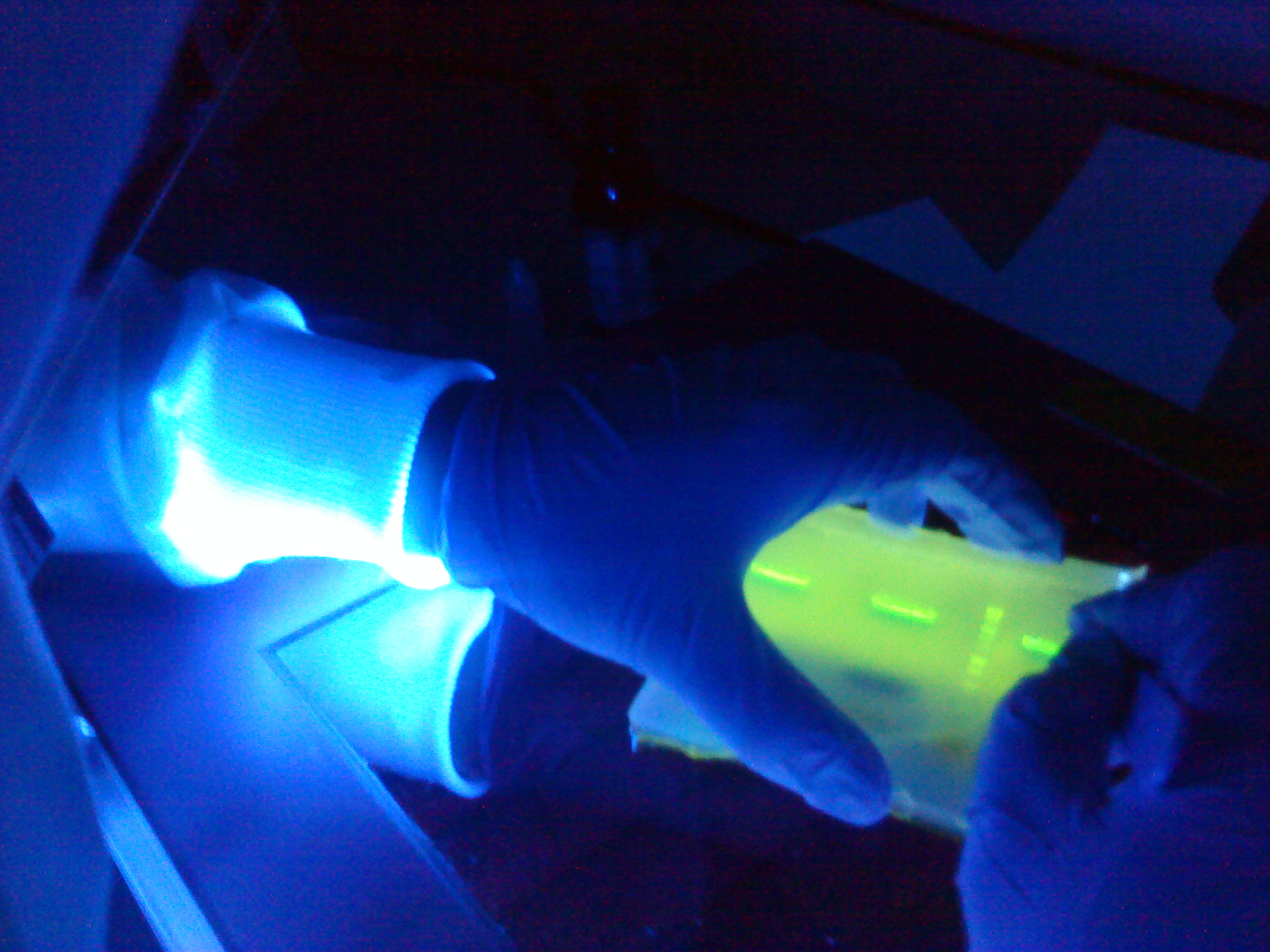
[[Image:Newcastle Gel Extraction of spaIFEG genes.JPG|200px|Gel Extraction of spaIFEG genes]] | [[Image:Newcastle Gel Extraction of spaIFEG genes.JPG|200px|Gel Extraction of spaIFEG genes]] | ||
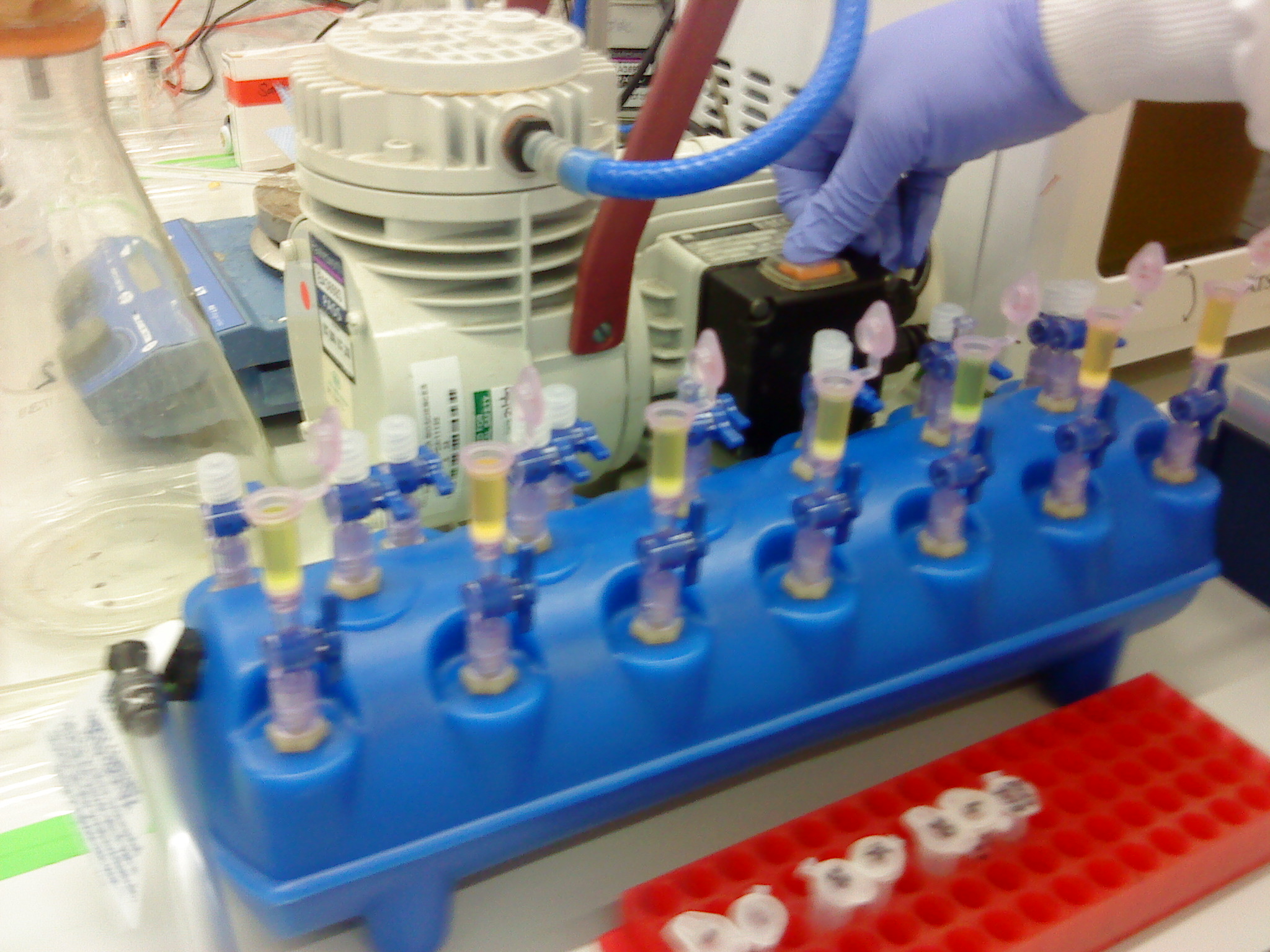
| - | + | [[Image:Newcastle Vacuum Manifold 1.JPG|150px|Setting up the tubes for the vacuum manifold]] | |
| - | [[Image:Newcastle Vacuum Manifold 1.JPG|150px|Setting up the tubes for the | + | [[Image:Newcastle Vacuum Manifold 2.JPG|150px|Setting up the tubes for the vacuum manifold]] |
| - | [[Image:Newcastle Vacuum Manifold 2.JPG|150px|Setting up the tubes for the | + | [[Image:Newcastle Vacuum Manifold 3.JPG|200px|Vacuum manifold with the QIAquick spin columns loaded with the samples]] |
| - | [[Image:Newcastle Vacuum Manifold 3.JPG|200px|Vacuum | + | |
| - | + | ||
==Results== | ==Results== | ||
| - | + | {|border=1 | |
| - | + | |- | |
| - | + | !'''Vector''' | |
| - | + | !'''Promoter''' | |
| - | + | !'''Immunity gene cluster''' | |
| + | !'''Double terminator''' | ||
| + | |- | ||
| + | |21.3 µl/ml | ||
| + | |28.8 µl/ml | ||
| + | |27.0 µl/ml | ||
| + | |27.5 µl/ml | ||
| + | |} | ||
| + | '''Table 1''': Nanodrop spectrophotometer results. Table represents the amount of plasmid present in µl/ml quantity. | ||
==Discussion== | ==Discussion== | ||
| - | + | The concentration of the fragments range from 21.3 µl/ml to 28.8 µl/ml. This is acceptable as during the gel extraction step, some of the DNA would be lost. | |
==Conclusion== | ==Conclusion== | ||
| - | + | We have successfully extracted all the parts required for the Subtilin Immunity BioBrick. The Gibson cloning method will be used next week. | |
Latest revision as of 23:41, 27 October 2010

| |||||||||||||
| |||||||||||||
Contents |
Gel electrophoresis of subtilin immunity part fragments
Aims
The aim of this experiment is to do gel extraction for the spaIFEG gene cluster amplifed on the 12th August, 2010 and the Plasmid Vector, Promoter & RBS and Double terminator amplifed on the 11th August, 2010. Finally, NanoDrop will be performed for all the fragments.
Materials and protocol
Please refer to the gel extraction and NanoDrop protocols. Instead of using the centrifuge to bind the DNA from our sample to the QIAquick column, a vacuum manifold was used instead. It is the first time it had been used. The advantage of this was that the extraction could be carried out all at the same time.
Results
| Vector | Promoter | Immunity gene cluster | Double terminator |
|---|---|---|---|
| 21.3 µl/ml | 28.8 µl/ml | 27.0 µl/ml | 27.5 µl/ml |
Table 1: Nanodrop spectrophotometer results. Table represents the amount of plasmid present in µl/ml quantity.
Discussion
The concentration of the fragments range from 21.3 µl/ml to 28.8 µl/ml. This is acceptable as during the gel extraction step, some of the DNA would be lost.
Conclusion
We have successfully extracted all the parts required for the Subtilin Immunity BioBrick. The Gibson cloning method will be used next week.
Go back to our main Lab book page
 
|
 "
"