|
|
| (183 intermediate revisions not shown) |
| Line 3: |
Line 3: |
| | | | |
| | <div id="tf_menu"> | | <div id="tf_menu"> |
| - | menu | + | <font size="5" color="#eb8300"><b><center>Project menu</center></b></font> |
| - | [[Team:Tokyo_Tech/Project/wolf_coli/New_Series_of_PompC|PompC]] | + | |
| | + | <center> |
| | + | <table id="table-01"> |
| | + | <tr> |
| | + | <td>[[Team:Tokyo_Tech|1 Graphic abstract]]<br> |
| | + | </td> |
| | + | </tr> |
| | + | <td>2 Apple reporter<br> |
| | + | :[[Team:Tokyo_Tech/Project/Apple_Reporter|2-1 Color]] |
| | + | :[[Team:Tokyo_Tech/Project/Apple_Reporter2|2-2 Fragrance]] |
| | + | </td> |
| | + | <tr> |
| | + | <td>[[Team:Tokyo_Tech/Project/Artificial_Cooperation_System|3 Artificial Cooperation System]]<br> |
| | + | :[[Team:Tokyo_Tech/Project/Artificial_Cooperation_System/lux_act_rep|3-1 lux activation/repression promoter]] |
| | + | :[[Team:Tokyo_Tech/Project/Artificial_Cooperation_System/Cm_assay|3-2 resistance gene activation device]] |
| | + | :[[Team:Tokyo_Tech/Project/Artificial_Cooperation_System/luxI_assay|3-3 ''lux''I Assay]] |
| | + | :[[Team:Tokyo_Tech/Project/Artificial_Cooperation_System/modeling|3-4 modeling]] |
| | + | </td> |
| | + | </tr> |
| | + | <tr> |
| | + | <th>4 Wolf coli overview -YOU ARE HERE!-<br> |
| | + | :[[Team:Tokyo_Tech/Project/wolf_coli/New_Series_of_PompC|4-1 New seriesof P''ompC'']] |
| | + | :[[Team:Tokyo_Tech/Project/wolf_coli/lacIM1|4-2 lacIM1 for band-detect network ]] |
| | + | :[[Team:Tokyo_Tech/Project/wolf_coli/System|4-3 Wolf coli system]] |
| | + | </th> |
| | + | </tr> |
| | + | </table> |
| | + | </center> |
| | + | |
| | | | |
| - | [[Team:Tokyo_Tech/Project/wolf_coli/lacIM1|lacIM1]]
| |
| | </div> <!-- tf_menu --> | | </div> <!-- tf_menu --> |
| | | | |
| - | <div id="tf_SubWrapper"> | + | <div id="tf_SubWrapper"> |
| - | =Wolf coli= | + | <font size="5"><b>4 Wolf coli Overview</b></font> |
| | ==Introduction== | | ==Introduction== |
| - |
| + | [[Image:tokyotech_wolfcoli_system_ver2.png|left|thumb|250px|Fig. 4-1 Cooperative activity of Artificial Cooperation System]] |
| - | In order to assemble a more intelligible& imaginable system, we linked our project to a well-known character ”Wolfman”.
| + | [[Image:tokyotech_wolfcoli_system_ver5.png|right|thumb|350px|Figure 4-2. Overview of “Wolf coli” system]] |
| - | In our attempt to produce “wolfcoli”, we introduced the Artificial Cooperation System.
| + | |
| - | A red-light-dependent gene expression system and synthetic band detector circuit were used to perform function designed.
| + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | + | |
| | | | |
| - | ==Highlight==
| |
| - | ・In order to design E. coli to have humanity, we attemped to integrate a red-light-dependent gene expression system and synthetic band detector circuit into the Artificial cooperation system.
| |
| | | | |
| - | ・We have constructed the NEW BioBrick series of ompC promoter .<br>
| |
| - | -We found that lacIM1 shows weaker repression than WT (Fig. 11).
| |
| | | | |
| - | ・We have characterized lacIM1(BBa_K082026) which is the key component in the synthetic band detector circuit. Though this part was registered by USTC(2008), it was not well characterized in Wiki.<br>
| |
| - | -We found that lacIM1 shows weaker repression than WT (Fig. 11).
| |
| | | | |
| - | ==Requirement==
| |
| - | ===Red-light-dependent gene expression system===
| |
| - | A red-light-dependent gene expression system has been introduced into E. coli(Anselm Levskaya et al.2005). And these BioBrick parts have been registered.
| |
| - | Photoreceptors are not found in E. coli. Then, they introduced a light sensor form a cyanobacterium into E. coli. The response regulator of phytochrome do not directly regulate gene expression, so they fused a cyanobacterial photoreceptor from Cph1 to an E. coli intracellular histidine kinase domain and response-regulator from EnvZ–OmpR. Moreover , Cph1–EnvZ chimaeras were then activated by introduction of two phycocyanobilin-biosynthesis genes that convert heme into phycocyanobilin.<br>
| |
| | | | |
| - | In low red light condition, Cph1–EnvZ chimeras are activated. EnvZ is autophosphorylated and passes phosphoryl group intramolecularly to OmpR. Then, phosphorylated OmpR binds to ompC promoter and activates the transcription of the downstream gene.<br>
| |
| - | In high red light condition, Cph1–EnvZ chimaeras are not activated. EnvZ is dephosphorylated, and thus result in no phosphorylation of OmpR. Then, OmpR can’ t bind to ompC promoter. The transcription of the downstream gene aren’t occur. <br>
| |
| | | | |
| - | What is important in this system is that light intensity is converted into concentration of phosphorylated OmpR.<br>
| |
| | | | |
| - | ===Synthetic band detector circuit===
| |
| - | Gene expresses only within the specific concentration range of chemical signals. Synthetic band detector circuit exhibits transient gene expression in response to concentration of chemical signals. (Subhayu Basu et al.2005)
| |
| - | Previously, USTC(2008) attempted to build this circuit and registered parts for this circuit.
| |
| | | | |
| - | In Basu’s group paper, LuxR, an AHL-dependent transcriptional regulator, was used. (Fig. 2)
| |
| - | LuxR activates the expression of cI and LacIM1, which has a lower affinity to lac promoter than WT. Therefore, LacIM1 shows weaker repression than WT.
| |
| | | | |
| - | High concentration of AHL(Fig. 3a) results in high levels of CI and LacIM1 and repression of GFP. <br>
| |
| - | At low concentration of AHL(Fig. 3b), Lac IM1 and CI are expressed only at basal levels. This enables the expression of a LacIWT, again resulting in GFP repression<br>
| |
| - | At intermediate concentration of AHL (Fig. 3c), it’s result in moderate levels of CI and LacIM1. However, because the repression efficiency of CI is significantly higher than that of LacIM1, CI effectively shuts off LacI expression while the LacIM1 concentration is below the threshold required to repress GFP production. This difference between the CI and LacIM1 repression efficiencies, in combination with a feed-forward loop that begins with LuxR and culminates in GFP, affords the circuit the desired non-monotonic response to AHL dosages.
| |
| | | | |
| | | | |
| - | ==Genetic circuit==
| |
| - | ===circuit===
| |
| | | | |
| | | | |
| - | We aimed to introduce a red-light-dependent gene expression system and synthetic band detector circuit into the Artificial Cooperation System. (Fig. 4)
| + | Have you heard the legend of 'The Wolfman'? They're ordinary man at daytime, but suddenly transform into a ferocious wolf in the full-moon night. Our project aim to imitate the character of Wolfman, more specifically, designing two types of ''E.coli'' that helps each other to survive at daytime, whereas competing at full moon night. In order to create the “Wolf coli”, we introduced " red-light-dependent gene expression network"[1] and "band-detect network"[2] into one cell, and combined these networks with the Artificial Cooperation System (Fig. 4-3). We characterized new series of ''OmpC'' promoter, and LacIM1 which are crucial parts of our networks. |
| - | Two systems were united by applying OmpR. OmpR usually works as regulative factor of red-light sensing system, and we utilized this to regulate synthetic band detector circuit. OmpR is a transcriptional regulator, which activates the expression of lambda repressor (CI) and Lac repressor (LacIM1, a product of a codon-modified lacI) in this system
| + | |
| | | | |
| - | ・At night (Low light intensity)
| + | [[Image:Tokyotech_ompc_graph.jpg|thumb|left|320px|Fig. 4-3 The induction of new P''ompC'' series in high osmolarity medium at 4 hours. This work is done by Thiprampai THAMAMONGOOD and Taichi NAKAMURA]] |
| - | In the dark, high concentrations of phosphorylated OmpR activates ompC promoter. That’s results in high cytoplasmic levels of CI and LacIM1 and repression of the inverter. Then, Artificial Cooperation System turns on, too.
| + | |
| | | | |
| | + | [[Image:Tokyotech_LacIM1_data.png|thumb|right|230px|Fig. 4-4 Repression efficiency of LacIM1 (BBa_K395401) / LacIWT (BBa_K395402) exposed to arabinose and IPTG. This work is done by Mitsuhiko ODERA]] |
| | | | |
| | | | |
| Line 72: |
Line 83: |
| | | | |
| | | | |
| - | ・At daytime (High light intensity)
| |
| - | Light drives the sensor to a state in which phosphorylation of OmpR is inhibited. There is no expression from ompC promoter. In low concentrations of phosphorylated OmpR, LacIM1 and CI are expressed only at basal levels. This enables the expression of a wild-type LacI, again resulting in repression of the inverter. Then, Artificial Cooperation System turns on , too.
| |
| - |
| |
| - | ・At full moon night (Intermediate light intensity)
| |
| - | Intermediate phosphorylated OmpR concentration results in moderate levels of CI and LacIM1. However, because the repression efficiency of CI is significantly higher than that of LacIM1, CI effectively shuts off LacI expression while the LacIM1 concentration is below the threshold required to repress the inverter. This difference between the CI and LacIM1 repression efficiencies drives the inverter to a state of turning on. It’s results in turning off the Artificial Cooperation System.
| |
| | | | |
| - | ・Seriese of PompC
| |
| - | In our system, transient gene expression need to occur only at light intensity of full-moon. Therefore, we must find an appropriate range of the light intensity. To solve this problem, we have the following approachs.
| |
| - | Ⅰ Modification of the chimaera protein(sensor protein, cph8) alters the ratio of phosphorylated OmpR, which is the transcriptional activator of the red-light dependent gene expression.
| |
| - | Ⅱ Modification of OmpR protein also alters the ratio of phosphorylated OmpR, which is the transcriptional activator of the red-light dependent gene expression.
| |
| - | Ⅲ Modification of ompC promoter alters the binding efficiency of phosphorylated OmpR.
| |
| | | | |
| - | As written above, there are various methods to integrate the two systems.
| |
| - | Among the approaches, modification of the Chimera protein and OmpR protein is very difficult.
| |
| - | Therefore, we made the series of ompC promoter.
| |
| - | 実験、詳細については↓で行ったので見ていってね。
| |
| | | | |
| | | | |
| Line 94: |
Line 91: |
| | | | |
| | | | |
| - | ・LacIM1
| |
| - | Intermediate phosphorylated OmpR concentrations result in moderate levels of CI and LacIM1. However, because the repression efficiency of CI is significantly higher than that of LacIM1, CI effectively shuts off LacI expression while the LacIM1 concentration is below the threshold required to repress the inverter. This difference between the CI and LacIM1 repression efficiencies can afford the circuit the desired non-monotonic response to phosphorylated OmpR concentration. Therefore, lacIM1 is a key component for building the synthetic band detector circuit. We characterize the function of this part (BBa_K082026) , which was designed by USTC (2008) .
| |
| - | 実験、詳細については↓で行ったので見ていってね。
| |
| | | | |
| - | ==Works==
| + | We have succeeded in designing 2 new osmoregulative promoters, P''ompC(CB)'' [http://partsregistry.org/Part:BBa_K395302 (BBa_K395302)] and P''ompC(CS1)''[http://partsregistry.org/Part:BBa_K395303 (BBa_K395303)], which can be utilized in the red-light-dependent gene expression network (Fig. 4-3). We also characterized LacIM1 [http://partsregistry.org/Part:BBa_K082026 (BBa_K082026)], a mutant of LacIWT,which is a key component in the band-detect network. Although, this part was registered by USTC(2008) [3], it was not well characterized in the BioBrick registry. We confirmed that LacIM1 shows weaker repression to Plac than its wild type. (Fig. 4-4) |
| - | ===Overview of new ''OmpC'' promoter series===
| + | |
| - | We constructed BioBrick parts of the new series of osmoregulative promoters which are derivatives of the wild type ''OmpC'' promoter. The new series of promoters are P''ompC(C)'', P''ompC(CB)'' and P''ompC(CS1)''. In order to measure the strength of each promoter, we used GFP as a reporter. We found that expression of GFP in ''OmpC(CB)'' and ''OmpC(CS1)'' promoters increased in high osmolarity medium comparing with the expression in low osmolarity medium. In contrast, under same conditions, there is no significant difference of GFP expression in ''OmpC(C)'' and ''OmpC(WT)'' promoters. | + | |
| | | | |
| - | *;New BioBrick Parts
| + | ==Wolf coli and Artificial Cooperative System == |
| - | [[Image:Tokyotech_ompc_graph.jpg|left|thumb|400px|Figure 1. The induction of new'' OmpC'' series in high osmolarity medium ]] | + | [[Image:tokyotech_wolfcoli_system_ver2.png|thumb|center|350px|Fig. 4-1 Cooperative activity of Artificial Cooperation System]] |
| | | | |
| | + | The Artificial Cooperation System was designed to be switched off during the “full moon night”. Therefore, Sympathetic coli would transform into Wolf coli at the “full moon light”. During this period, communication between 2 types of cells doesn't occur. Hence, both types of cells are unable to recognize each other by quorum sensing and become competitors. Cooperative activity in Artificial Cooperation System is regulated by 3 levels of light intensity which are weak, medium and strong. “Weak light” from the crescent moon switches the Artificial Cooperation System on, thus two types of cells are able to communicate and help each other. “Medium light” during the full moon night can switch the system off resulting in appearance of the “Wolfcoli”. During the daytime, “strong light” from the sun activates the Artificial Cooperation System. |
| | | | |
| - |
| + | ==P''OmpC'' in red-light-dependent gene expression network== |
| | + | [[Image:Tokyotech wolfcoli system_ver5.png|left|thumb|300px|Fig. 4-5 Overview of red-light-dependent gene expression network in Wolf coli system]] |
| | + | [[Image:Tokyotech_ompc_graph.jpg|thumb|left|320px|Fig. 4-6 The activation of new P''ompC'' series in high osmolarity medium at 4 hours. This work is done by Thiprampai THAMAMONGOOD and Taichi NAKAMURA]] |
| | | | |
| | | | |
| | + | ''OmpC'' promoter, in red-light-dependent gene expression network, plays a crucial role in initiating the transformation of Sympathetic coli into Wolf coli. To accomplish band-detect circuit in light sensing system, varieties of ''OmpC'' promoters were designed and characterized so as to find an appropriate strength of the promoter particularly which can be activated by the “full moon light”.(Fig.4-5) |
| | + | [https://2010.igem.org/Team:Tokyo_Tech/Project/wolf_coli/New_Series_of_PompC#Introduction ...see more about ''OmpC'' promoter ](Fig. 4-6) |
| | | | |
| | + | ==LacIMI in band-detect network== |
| | + | [[image:Tokyotech_band_detect.png|340px|thumb|left| Fig.4-7 Patial circuit of band-detect network ]] |
| | + | [[Image:Tokyotech_LacIM1_data.png|thumb|right|240px|Fig. 4-8.Repression efficiency of LacIM1 (BBa_K395401) / LacIWT (BBa_K395402) exposed to arabinose and IPTG. This work is done by Mitsuhiko ODERA]] |
| | | | |
| | | | |
| Line 128: |
Line 128: |
| | | | |
| | | | |
| - | We succeeded in designing 2 news osmoregulative promoters, P''OmpC(CB)'' and P''OmpC(CS1)'', which can also be utilized in light sensitive system.
| |
| - | [https://2010.igem.org/Team:Tokyo_Tech/Project/wolf_coli/New_Series_of_PompC ...see more about P''ompC'']
| |
| | | | |
| - | ===Overview of characterozation of ''LacIM1'' ===
| |
| - | LacIM1 is a key component in the synthetic band detector circuit. Thus, we characterize the function of this part (BBa_K082026), which is designed by USTC(2008) . In order to measure the function of lacI proteins, we constructed following two plasmids, BBa_K395401, and BBa_K395402, which have an arabinose inducible promoter(Fig. 10). We measured GFP expression dependent on the input of arabinose and IPTG. For assay, we introduced two plasmids into DH5α. LacI proteins produced by arabinose induction represses GFP expression. The repression by lacI prorteins can be inhibited by IPTG induction. <br>
| |
| | | | |
| - | Results : We found that lacIM1 shows weaker repression than WT (Fig. 11).
| |
| - | This is the result of LacIM1.
| |
| - | In the absence of both arabinose and IPTG, GFP expression is about 1.8-folds stronger than that of the presence of arabinose and the absence of IPTG. We found that GFP expression is dependent on product of lacIM1 by arabinose induction. When there was LacIM1 by arabinose induction, GFP expression was repressed. That’s result shows that this repression is effect of LacIM1. This repression is dependent on product of lacIM1 by arabinose induction Therefore, This shows that this repression is the function of LacIM1. Increase of GFP production by addition of IPTG, with or without arabinose induction, shows the repressions are dependent on LacIM1. Without arabinose, increase of GFP production by addition of IPTG is leaky expression of LacIM1. This leaky expression could also explain the result of LacWT.(See more…)
| |
| | | | |
| | | | |
| | | | |
| | | | |
| | + | The band-detect network exhibits transient gene expression in response to concentration of chemical signals(Fig.4-7). In the band-detect network, LacIM1 is a crucial component due to its low repression efficiency[1]. According to the assay results, we confirmed that LacIM1 shows weaker repression to Plac than its wild type.(Fig. 4-8). [https://2010.igem.org/Team:Tokyo_Tech/Project/wolf_coli/lacIM1 …see more about lacIM1]. |
| | + | ==References== |
| | + | [1]. Basu S, Gerchman Y, Collins CH, et al. A synthetic multicellular system for programmed pattern formation. NATURE 2005;434,1130-1134 |
| | | | |
| | <!-- ここまでね--> | | <!-- ここまでね--> |
4 Wolf coli Overview
Introduction
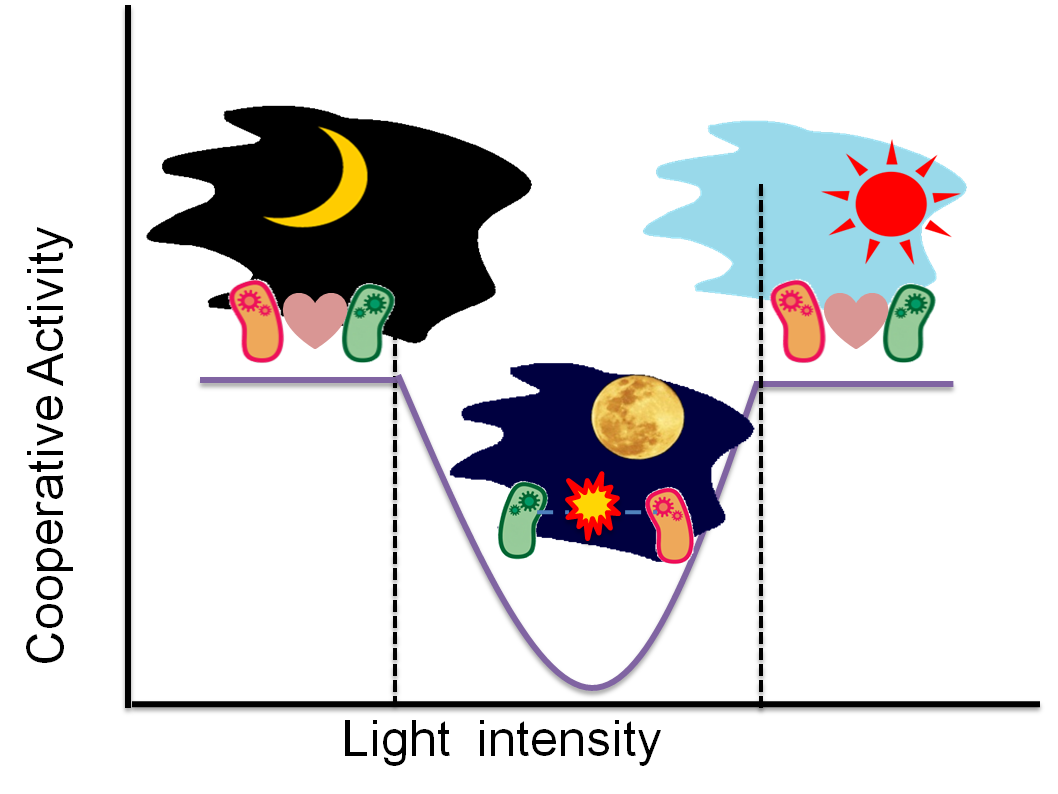
Fig. 4-1 Cooperative activity of Artificial Cooperation System
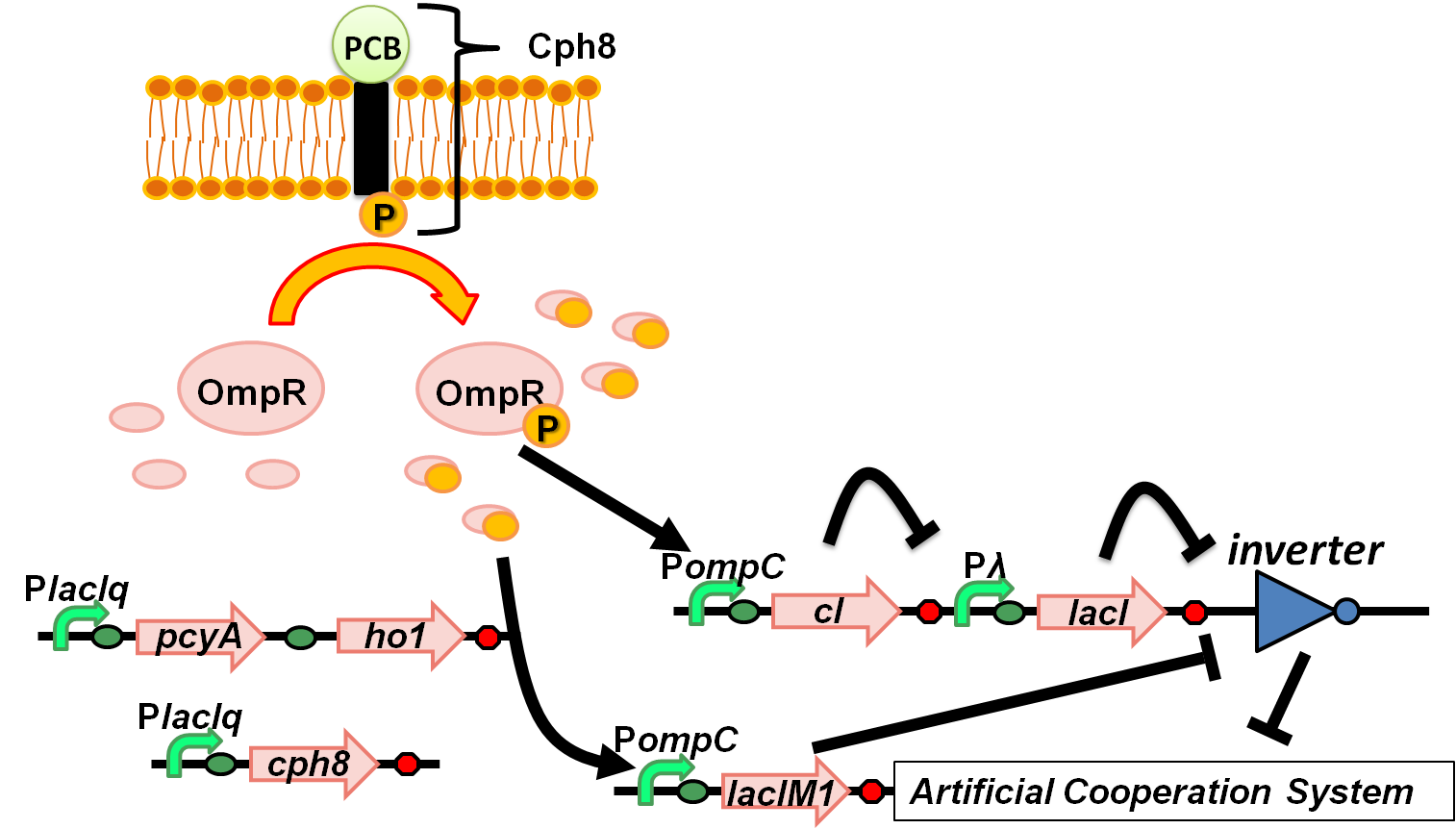
Figure 4-2. Overview of “Wolf coli” system
Have you heard the legend of 'The Wolfman'? They're ordinary man at daytime, but suddenly transform into a ferocious wolf in the full-moon night. Our project aim to imitate the character of Wolfman, more specifically, designing two types of E.coli that helps each other to survive at daytime, whereas competing at full moon night. In order to create the “Wolf coli”, we introduced " red-light-dependent gene expression network"[1] and "band-detect network"[2] into one cell, and combined these networks with the Artificial Cooperation System (Fig. 4-3). We characterized new series of OmpC promoter, and LacIM1 which are crucial parts of our networks.
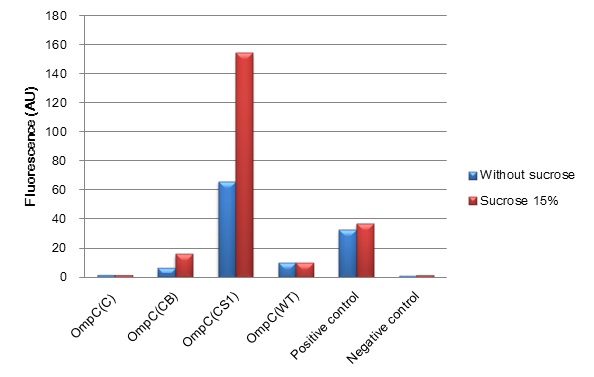
Fig. 4-3 The induction of new P
ompC series in high osmolarity medium at 4 hours. This work is done by Thiprampai THAMAMONGOOD and Taichi NAKAMURA
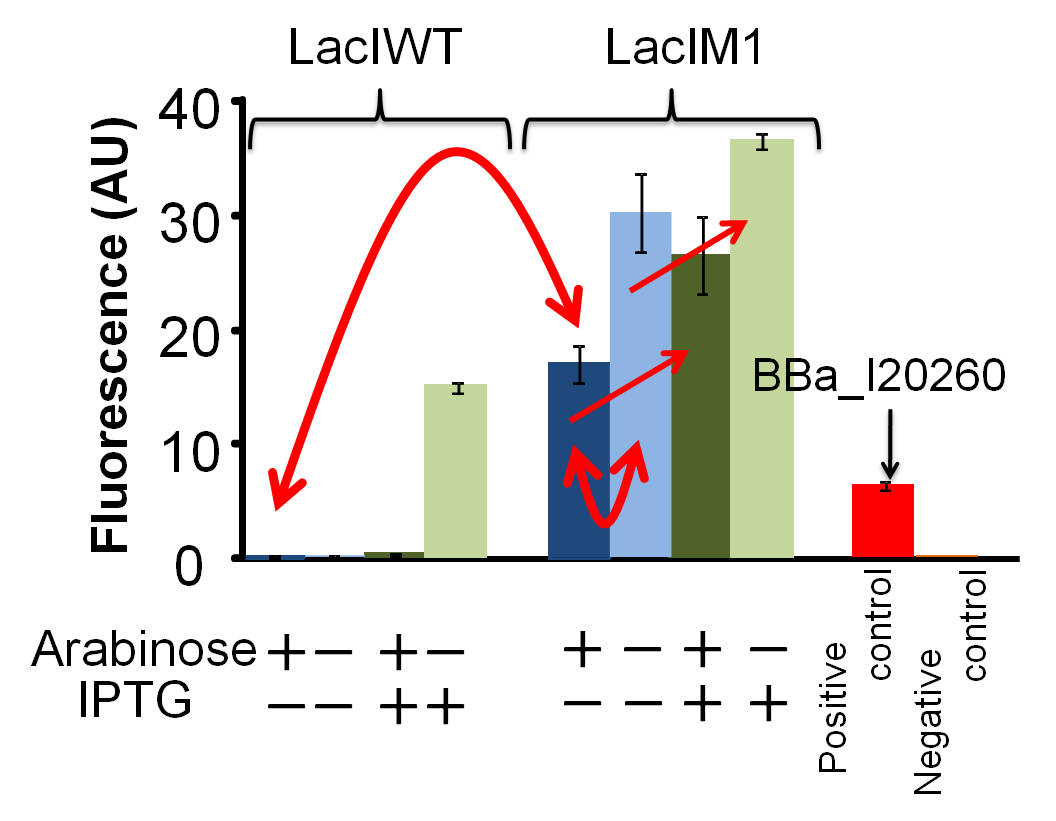
Fig. 4-4 Repression efficiency of LacIM1 (BBa_K395401) / LacIWT (BBa_K395402) exposed to arabinose and IPTG. This work is done by Mitsuhiko ODERA
We have succeeded in designing 2 new osmoregulative promoters, PompC(CB) [http://partsregistry.org/Part:BBa_K395302 (BBa_K395302)] and PompC(CS1)[http://partsregistry.org/Part:BBa_K395303 (BBa_K395303)], which can be utilized in the red-light-dependent gene expression network (Fig. 4-3). We also characterized LacIM1 [http://partsregistry.org/Part:BBa_K082026 (BBa_K082026)], a mutant of LacIWT,which is a key component in the band-detect network. Although, this part was registered by USTC(2008) [3], it was not well characterized in the BioBrick registry. We confirmed that LacIM1 shows weaker repression to Plac than its wild type. (Fig. 4-4)
Wolf coli and Artificial Cooperative System

Fig. 4-1 Cooperative activity of Artificial Cooperation System
The Artificial Cooperation System was designed to be switched off during the “full moon night”. Therefore, Sympathetic coli would transform into Wolf coli at the “full moon light”. During this period, communication between 2 types of cells doesn't occur. Hence, both types of cells are unable to recognize each other by quorum sensing and become competitors. Cooperative activity in Artificial Cooperation System is regulated by 3 levels of light intensity which are weak, medium and strong. “Weak light” from the crescent moon switches the Artificial Cooperation System on, thus two types of cells are able to communicate and help each other. “Medium light” during the full moon night can switch the system off resulting in appearance of the “Wolfcoli”. During the daytime, “strong light” from the sun activates the Artificial Cooperation System.
POmpC in red-light-dependent gene expression network

Fig. 4-5 Overview of red-light-dependent gene expression network in Wolf coli system

Fig. 4-6 The activation of new P
ompC series in high osmolarity medium at 4 hours. This work is done by Thiprampai THAMAMONGOOD and Taichi NAKAMURA
OmpC promoter, in red-light-dependent gene expression network, plays a crucial role in initiating the transformation of Sympathetic coli into Wolf coli. To accomplish band-detect circuit in light sensing system, varieties of OmpC promoters were designed and characterized so as to find an appropriate strength of the promoter particularly which can be activated by the “full moon light”.(Fig.4-5)
...see more about OmpC promoter (Fig. 4-6)
LacIMI in band-detect network
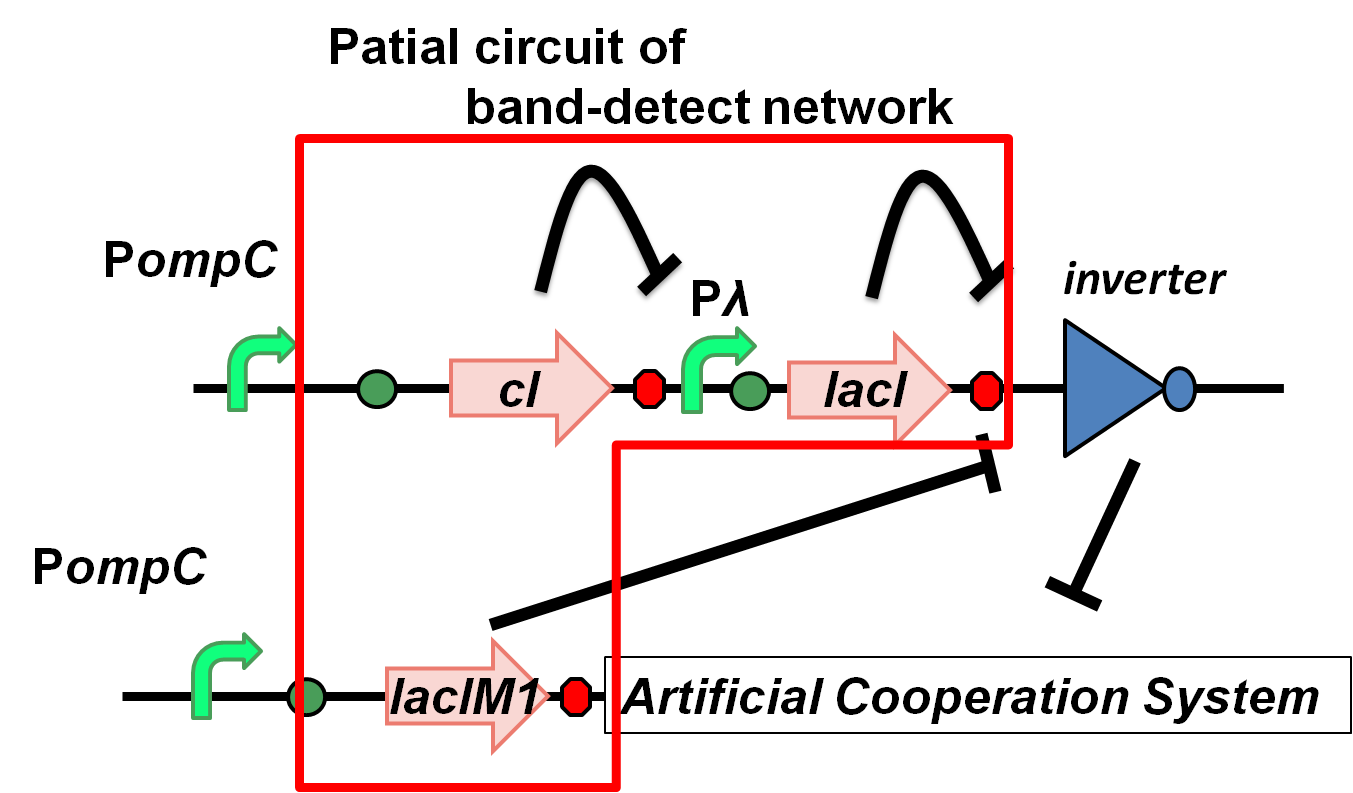
Fig.4-7 Patial circuit of band-detect network

Fig. 4-8.Repression efficiency of LacIM1 (BBa_K395401) / LacIWT (BBa_K395402) exposed to arabinose and IPTG. This work is done by Mitsuhiko ODERA
The band-detect network exhibits transient gene expression in response to concentration of chemical signals(Fig.4-7). In the band-detect network, LacIM1 is a crucial component due to its low repression efficiency[1]. According to the assay results, we confirmed that LacIM1 shows weaker repression to Plac than its wild type.(Fig. 4-8). …see more about lacIM1.
References
[1]. Basu S, Gerchman Y, Collins CH, et al. A synthetic multicellular system for programmed pattern formation. NATURE 2005;434,1130-1134
 "
"




