Team:Tokyo Tech/Project/Apple Reporter2
From 2010.igem.org
(→Result) |
(→Reference) |
||
| (33 intermediate revisions not shown) | |||
| Line 8: | Line 8: | ||
<table id="table-01"> | <table id="table-01"> | ||
<tr> | <tr> | ||
| - | <td>[[Team:Tokyo_Tech|1 | + | <td>[[Team:Tokyo_Tech|1 Graphic abstract]]<br> |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 25: | Line 25: | ||
<tr> | <tr> | ||
<td>[[Team:Tokyo_Tech/Project/wolf_coli|4 wolfcoli overview]]<br> | <td>[[Team:Tokyo_Tech/Project/wolf_coli|4 wolfcoli overview]]<br> | ||
| - | :[[Team:Tokyo_Tech/Project/wolf_coli/New_Series_of_PompC|4-1 | + | :[[Team:Tokyo_Tech/Project/wolf_coli/New_Series_of_PompC|4-1 New seriesof P''ompC'']] |
:[[Team:Tokyo_Tech/Project/wolf_coli/lacIM1|4-2 lacIM1 for band-detect network ]] | :[[Team:Tokyo_Tech/Project/wolf_coli/lacIM1|4-2 lacIM1 for band-detect network ]] | ||
| - | :[[Team:Tokyo_Tech/Project/wolf_coli/System|4-3 | + | :[[Team:Tokyo_Tech/Project/wolf_coli/System|4-3 Wolf coli system]] |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 40: | Line 40: | ||
<font size="5"><b>2-2 Fragrance</b></font> | <font size="5"><b>2-2 Fragrance</b></font> | ||
=Abstract= | =Abstract= | ||
| + | We designed apple fragrance expression device with MpAAT1<sup>[1]</sup>. MpAAT1 is able to produce ester compounds with apple fragrance using alcohols and Acetyl-CoA. Fig. 2-2-1 shows the outline of the device. We performed gas chromatography to confirm the product of esters. The results revealed that MpAAT1 could produce 2-methylbutyl acetate and butyl acetate using 2-methyl butanol and butanol respectively (Fig. 2-2-2). 2-methylbutyl acetate and butyl acetate are known as the Apple fragrance. | ||
| - | |||
| - | [[image:Tokyotech apple fragrance device.png|630px|center|thumb|Fig. 2-2-1. Apple fragrance expression device.]] | + | [[image:Tokyotech apple fragrance device.png|630px|center|thumb|Fig. 2-2-1. Apple fragrance expression device. <Br>Abbreviations : 2-methyl butanol (2-MeBuOH), butanol (BuOH), 2-methylbutyl acetate (2-MeBuOAc), butyl acetate (BuOAc).]] |
| - | |||
| - | [[image: | + | [[image:Tokyotech_gas_chromatograpy.png|630px|center|thumb| Fig. 2-2-2. Gas chromatography analysis of apple fragrance expression device. <br>(1) MpAAT1 + BuOH, (2) No MpAAT1 + BuOAc, (3) No MpAAT1 + BuOH, (4) MpAAT1 + 2-MeBuOH, (5) No MpAAT1 + 2-MeBuOAc, (6) No MpAAT1 + 2-MeBuOH, (7) MpAAT1 - 2-MeBuOH.<br>This work is done by Toshitaka Matsubara.]] |
=Introduction= | =Introduction= | ||
| - | ''MpAAT1'' gene was isolated from ''Malus pumila'' ( | + | ''MpAAT1'' gene was isolated from ''Malus pumila'' (popular apple)<sup>[2]</sup>. This gene is expressed in leaves, flowers and fruit of apple. The recombinant enzyme (MpAAT1) to produce esters involved in apple fragrance, utilize various alcohol as substrates such as straight chain (C3–C10), branched chain, aromatic and terpene alcohols. In addition, various kinds of CoA derivatives, such as acetyl-CoA, are used for producing esters by MpAAT1. Both alcohol and CoA derivative are required for successful ester synthesis. The major components involved in apple fragrance are butyl acetate and 2-methylbutyl acetate<sup>[3]</sup>. We engineered apple fragrance expression device to produce these molecules by MpAAT1. This device is used as a reporter in Artificial Cooperation System of our projects, when dying cells are recued by its counterpart, it gives an apple fragrance for a token of their gratitude. |
=Result= | =Result= | ||
| - | We transformed MpAAT1([http://partsregistry.org/Part:BBa_K395602 BBa_K395602]) on pSB6A1 along with pTrx6 into ''E.coli'' BL21 DE3, and cultured after addition of alcohols(2-MeBuOH or BuOH) as substrates. | + | We transformed MpAAT1([http://partsregistry.org/Part:BBa_K395602 BBa_K395602]) on pSB6A1 along with pTrx6 into ''E.coli'' BL21 (DE3), and cultured after addition of alcohols(2-MeBuOH or BuOH) as substrates. |
| - | After 12 hours of incubation, we extracted organic | + | After 12 hours of incubation, we extracted organic solution layer from the culture and analyzed by gas chromatography (Fig. 2-2-2). Peaks of the esters producing apple fragrance (2-MeBuOAc or BuOAc) were detected. |
| - | [[image: | + | [[image:Tokyotech_gas_chromatograpy.png|630px|left|thumb|Fig. 2-2-2. Gas chromatography analysis of apple fragrance expression device. <br>(1) MpAAT1 + BuOH, (2) No MpAAT1 + BuOAc, (3) No MpAAT1 + BuOH, (4) MpAAT1 + 2-MeBuOH, (5) No MpAAT1 + 2-MeBuOAc, (6) No MpAAT1 + 2-MeBuOH, (7) MpAAT1 - 2-MeBuOH. |
<br>This work is done by Toshitaka Matsubara.]] | <br>This work is done by Toshitaka Matsubara.]] | ||
| - | ( | + | By comparing (1) with (2), BuOAc was confirmed when ''E. coli'' has MpAAT1. Moreover, by Comparing (1) with (3), BuOH (substrate) doesn’t contain BuOAc as impurity. By the same token, 2-MeBuOAc was confirmed when ''E. coli'' has MpAAT1 by comparing (4) with (5). Moreover, by Comparing (4) with (6), 2-MeBuOH (substrate) doesn’t contain 2-MeBuOAc as impurity. (7) shows ''E. coli'' which has MpAAT1 is able to convert alcohols (substrates) into fragrance molecules grown in no substrate culture. From these result, ''E. coli'' which has MpAAT1 successfully was able to produce 2-methylbutyl acetate and butyl acetate using 2-methyl butanol and butanol respectively. |
| - | + | ||
| - | + | ||
| - | ( | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | ( | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | From these | + | |
=Discussion= | =Discussion= | ||
| - | From the result of the experiment above, we can conclude that | + | From the result of the experiment above, we can conclude that apple fragrance expression device was able to produce esters using alcohols added as substrates. |
| - | Moreover, we synthesized MpAAT1 and esters using ''E.coli'' BL21 DE3 as a chassis, which was reported to be implausible in former report | + | Moreover, we synthesized MpAAT1 and esters using ''E.coli'' BL21 (DE3) as a chassis, which was reported to be implausible in former report<sup>[1]</sup>. |
In 2008, C.R. Shen and J.C. Liao<sup>[4]</sup> succeeded in synthesizing butanol from ''E.coli''. If we take advantage of this engineered ''E.coli'', we could produce apple fragrance ester without the addition of substrate. | In 2008, C.R. Shen and J.C. Liao<sup>[4]</sup> succeeded in synthesizing butanol from ''E.coli''. If we take advantage of this engineered ''E.coli'', we could produce apple fragrance ester without the addition of substrate. | ||
| - | = | + | =Material and Method= |
| - | Strains of ''E. coli''<Br>''E. coli'' | + | '''Strains of ''E. coli''''' |
| + | <Br>''E. coli'' BL21 (DE3) | ||
| - | Varieties of plasmid<Br> | + | |
| + | '''Varieties of plasmid'''<Br> | ||
MpAAT1 on pSB6A1<Br> | MpAAT1 on pSB6A1<Br> | ||
Trx on pACYC184 | Trx on pACYC184 | ||
| - | Substrate<Br> | + | |
| + | '''Substrate'''<Br> | ||
Butanol (final 0.4%)<Br> | Butanol (final 0.4%)<Br> | ||
2-methyl butanol (final 0.2%)<Br> | 2-methyl butanol (final 0.2%)<Br> | ||
| - | Inducer<Br> | + | '''Inducer'''<Br> |
| - | 100 mM IPTG<Br> | + | 100 mM IPTG (final 100 μM)<Br> |
| - | 20% arabinose<Br> | + | 20% arabinose (final 0.1%)<Br> |
| - | + | ||
| + | '''Internal standard solution'''<Br> | ||
Undecane solution: undecane 10 μL + ether 990 μL | Undecane solution: undecane 10 μL + ether 990 μL | ||
| - | MpAAT1 expression | + | '''MpAAT1 expression vector. '''<Br> |
| - | + | The coding sequence of ''MpAAT1'' gene was synthesized and optimized sequence by Mr.Gene. <Br> | |
| - | This artificial gene was | + | This artificial gene was ligated into vector pSB6A1 as MpAAT1 expression vector.<Br> |
Moreover, we introduced pTrx6 into this expression plasmid to stabilize the ''MpAAT1'' gene product.<Br> | Moreover, we introduced pTrx6 into this expression plasmid to stabilize the ''MpAAT1'' gene product.<Br> | ||
| - | MpAAT1 over expression conditions<Br> | + | |
| + | '''MpAAT1 over expression conditions'''<Br> | ||
Artificial gene has T7 promoter on the upstream of ''MpAAT1''.<Br> | Artificial gene has T7 promoter on the upstream of ''MpAAT1''.<Br> | ||
This promoter works by taking over T7 RNA polymerase from ''E. coli''.<Br> | This promoter works by taking over T7 RNA polymerase from ''E. coli''.<Br> | ||
| - | Therefore we utilized ''E. coli'' BL21 DE3 which has T7 RNA polymerase.<Br> | + | Therefore we utilized ''E. coli'' BL21 (DE3) which has T7 RNA polymerase.<Br> |
Furthermore, arabinose was added in culture to induce Trx which has arabinose-induced promoter.<Br> | Furthermore, arabinose was added in culture to induce Trx which has arabinose-induced promoter.<Br> | ||
| - | |||
| - | |||
| - | |||
| + | '''Cultivation''' <Br> | ||
| + | In order to express MpAAT1 and Trx in ''E. coli'' BL21 (DE3), we added 3 μL of 100 mM IPTG and 15 μL of 20% arabinose in 3ml LB culture. <Br> | ||
| - | Expression of MpAAT1 recombinant protein in ''E. coli'' | + | '''Expression of MpAAT1 recombinant protein in ''E. coli'''''<Br> |
| - | + | The cells were grown over night. The microbial solution was diluted 100-folds and grown for 2 hours in a fresh culture. After grown until the O.D. becomes 0.1, substrate and antibiotic, and inducer were added into the culture. This was grown overnight again. | |
| - | + | After the incubation, we centrifuged the solution (7000 × ''g'', 3 min) and collected the supernatant. | |
| - | + | Then, ether was used to separate oil through liquid-liquid solution. (shake supernatant solution 0.5 mL, with ether 0.5 mL and undecane solution). Finally, the oil layer was collected and analyzed by gas chromatography. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | '''Gas Chromatography analysis'''<Br> | |
| + | Gas Chromatography : SHIMADZU GAS CHROMATOGRAPH GC-14B<Br> | ||
Column: J&W SCIENTIFIC, DB-17, Film thickness 0.25 μm, Column Dimensions 15 m × 0.320 mm, Temperature Limits 40°C to 280°C (300°C Program)<Br> | Column: J&W SCIENTIFIC, DB-17, Film thickness 0.25 μm, Column Dimensions 15 m × 0.320 mm, Temperature Limits 40°C to 280°C (300°C Program)<Br> | ||
Conditions: column temperature 35°C, injector temperature 180°C, detector temperature 180°C<Br> | Conditions: column temperature 35°C, injector temperature 180°C, detector temperature 180°C<Br> | ||
Sample was injected 5 μL.<Br> | Sample was injected 5 μL.<Br> | ||
| - | = | + | =Reference= |
| - | [1] | + | [1] Edwige J. F. Souleyre, FEBS Journal, 272, 3132–3144 (2005)<Br> |
| - | [2] | + | [2] Young H, ''J Sci Food Agric'', 71, 329-336 (1996)<Br> |
[3] [http://www.ncbi.nlm.nih.gov/nucleotide/52139952 GenBank accession number AY707098]<Br> | [3] [http://www.ncbi.nlm.nih.gov/nucleotide/52139952 GenBank accession number AY707098]<Br> | ||
| - | [4] | + | [4] C.R. Shen, Metabolic Engineering, 10, 312–320 (2008)<Br> |
| - | + | ||
<!-- ここまでね--> | <!-- ここまでね--> | ||
</div> <!-- end SubWrapper --> | </div> <!-- end SubWrapper --> | ||
Latest revision as of 06:50, 18 November 2010
2-2 Fragrance
Contents |
Abstract
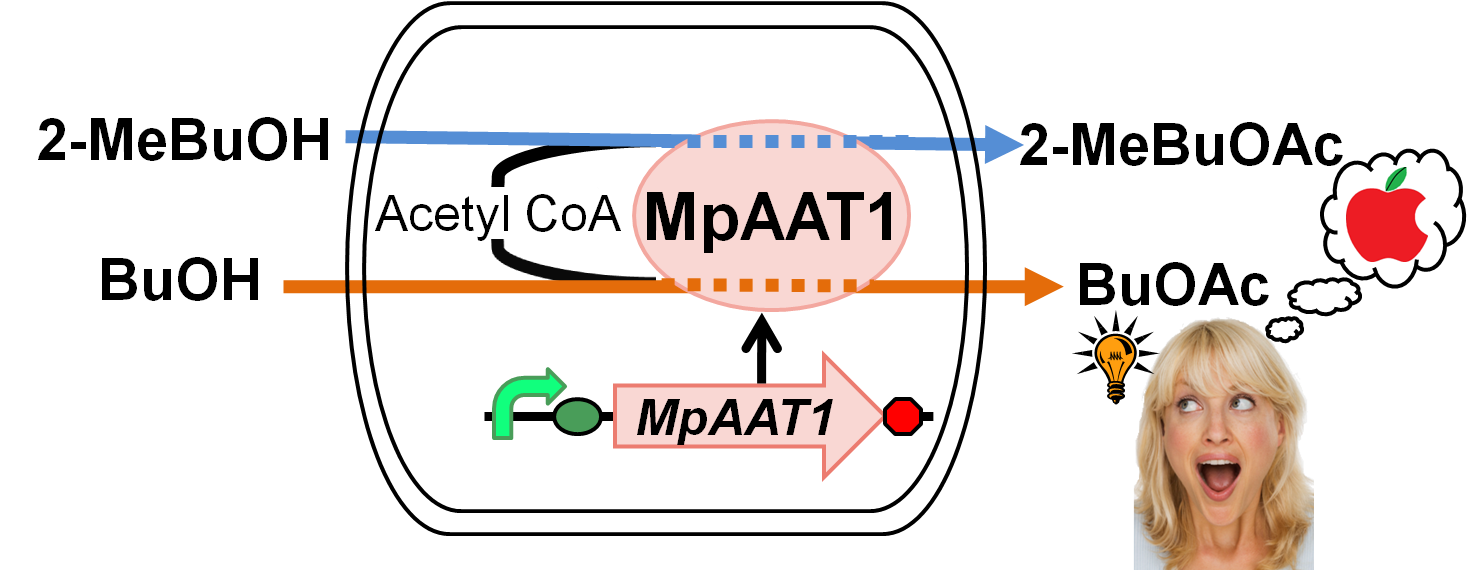
We designed apple fragrance expression device with MpAAT1[1]. MpAAT1 is able to produce ester compounds with apple fragrance using alcohols and Acetyl-CoA. Fig. 2-2-1 shows the outline of the device. We performed gas chromatography to confirm the product of esters. The results revealed that MpAAT1 could produce 2-methylbutyl acetate and butyl acetate using 2-methyl butanol and butanol respectively (Fig. 2-2-2). 2-methylbutyl acetate and butyl acetate are known as the Apple fragrance.
Introduction
MpAAT1 gene was isolated from Malus pumila (popular apple)[2]. This gene is expressed in leaves, flowers and fruit of apple. The recombinant enzyme (MpAAT1) to produce esters involved in apple fragrance, utilize various alcohol as substrates such as straight chain (C3–C10), branched chain, aromatic and terpene alcohols. In addition, various kinds of CoA derivatives, such as acetyl-CoA, are used for producing esters by MpAAT1. Both alcohol and CoA derivative are required for successful ester synthesis. The major components involved in apple fragrance are butyl acetate and 2-methylbutyl acetate[3]. We engineered apple fragrance expression device to produce these molecules by MpAAT1. This device is used as a reporter in Artificial Cooperation System of our projects, when dying cells are recued by its counterpart, it gives an apple fragrance for a token of their gratitude.
Result
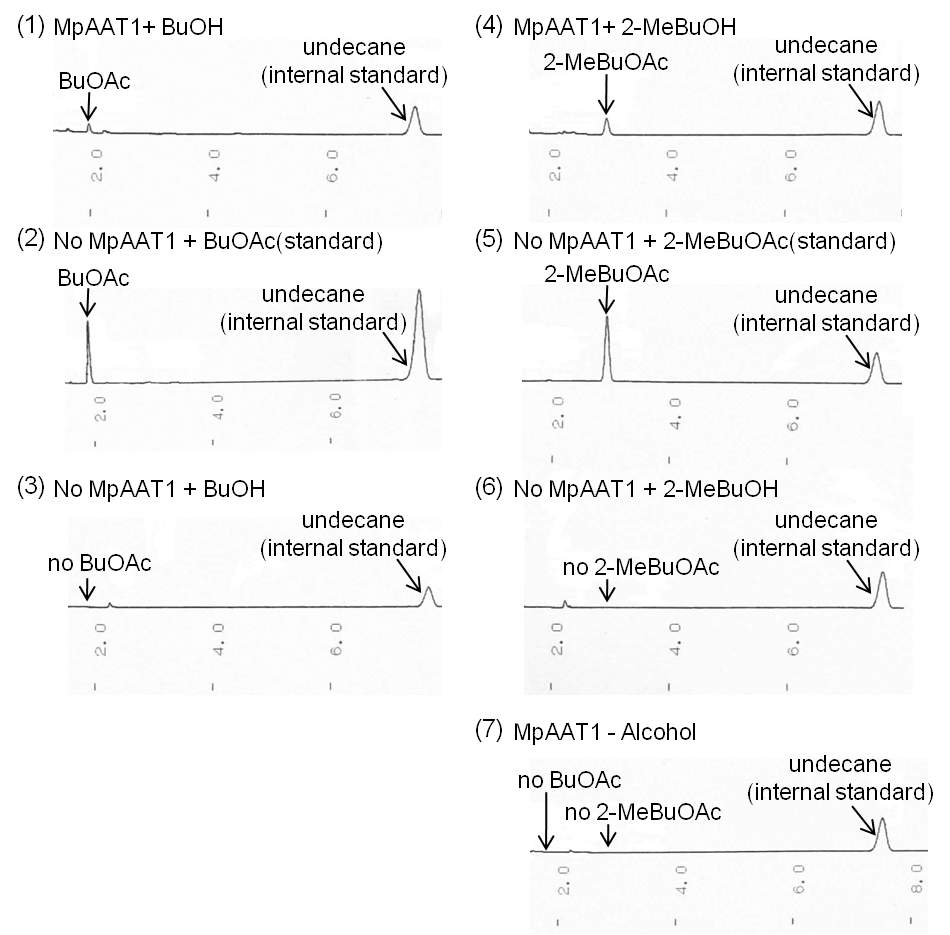
We transformed MpAAT1([http://partsregistry.org/Part:BBa_K395602 BBa_K395602]) on pSB6A1 along with pTrx6 into E.coli BL21 (DE3), and cultured after addition of alcohols(2-MeBuOH or BuOH) as substrates. After 12 hours of incubation, we extracted organic solution layer from the culture and analyzed by gas chromatography (Fig. 2-2-2). Peaks of the esters producing apple fragrance (2-MeBuOAc or BuOAc) were detected.
By comparing (1) with (2), BuOAc was confirmed when E. coli has MpAAT1. Moreover, by Comparing (1) with (3), BuOH (substrate) doesn’t contain BuOAc as impurity. By the same token, 2-MeBuOAc was confirmed when E. coli has MpAAT1 by comparing (4) with (5). Moreover, by Comparing (4) with (6), 2-MeBuOH (substrate) doesn’t contain 2-MeBuOAc as impurity. (7) shows E. coli which has MpAAT1 is able to convert alcohols (substrates) into fragrance molecules grown in no substrate culture. From these result, E. coli which has MpAAT1 successfully was able to produce 2-methylbutyl acetate and butyl acetate using 2-methyl butanol and butanol respectively.
Discussion
From the result of the experiment above, we can conclude that apple fragrance expression device was able to produce esters using alcohols added as substrates. Moreover, we synthesized MpAAT1 and esters using E.coli BL21 (DE3) as a chassis, which was reported to be implausible in former report[1]. In 2008, C.R. Shen and J.C. Liao[4] succeeded in synthesizing butanol from E.coli. If we take advantage of this engineered E.coli, we could produce apple fragrance ester without the addition of substrate.
Material and Method
Strains of E. coli
E. coli BL21 (DE3)
Varieties of plasmid
MpAAT1 on pSB6A1
Trx on pACYC184
Substrate
Butanol (final 0.4%)
2-methyl butanol (final 0.2%)
Inducer
100 mM IPTG (final 100 μM)
20% arabinose (final 0.1%)
Internal standard solution
Undecane solution: undecane 10 μL + ether 990 μL
MpAAT1 expression vector.
The coding sequence of MpAAT1 gene was synthesized and optimized sequence by Mr.Gene.
This artificial gene was ligated into vector pSB6A1 as MpAAT1 expression vector.
Moreover, we introduced pTrx6 into this expression plasmid to stabilize the MpAAT1 gene product.
MpAAT1 over expression conditions
Artificial gene has T7 promoter on the upstream of MpAAT1.
This promoter works by taking over T7 RNA polymerase from E. coli.
Therefore we utilized E. coli BL21 (DE3) which has T7 RNA polymerase.
Furthermore, arabinose was added in culture to induce Trx which has arabinose-induced promoter.
Cultivation
In order to express MpAAT1 and Trx in E. coli BL21 (DE3), we added 3 μL of 100 mM IPTG and 15 μL of 20% arabinose in 3ml LB culture.
Expression of MpAAT1 recombinant protein in E. coli
The cells were grown over night. The microbial solution was diluted 100-folds and grown for 2 hours in a fresh culture. After grown until the O.D. becomes 0.1, substrate and antibiotic, and inducer were added into the culture. This was grown overnight again.
After the incubation, we centrifuged the solution (7000 × g, 3 min) and collected the supernatant.
Then, ether was used to separate oil through liquid-liquid solution. (shake supernatant solution 0.5 mL, with ether 0.5 mL and undecane solution). Finally, the oil layer was collected and analyzed by gas chromatography.
Gas Chromatography analysis
Gas Chromatography : SHIMADZU GAS CHROMATOGRAPH GC-14B
Column: J&W SCIENTIFIC, DB-17, Film thickness 0.25 μm, Column Dimensions 15 m × 0.320 mm, Temperature Limits 40°C to 280°C (300°C Program)
Conditions: column temperature 35°C, injector temperature 180°C, detector temperature 180°C
Sample was injected 5 μL.
Reference
[1] Edwige J. F. Souleyre, FEBS Journal, 272, 3132–3144 (2005)
[2] Young H, J Sci Food Agric, 71, 329-336 (1996)
[3] [http://www.ncbi.nlm.nih.gov/nucleotide/52139952 GenBank accession number AY707098]
[4] C.R. Shen, Metabolic Engineering, 10, 312–320 (2008)
 "
"