Team:Newcastle/18 August 2010
From 2010.igem.org
RachelBoyd (Talk | contribs) (New page: {{Team:Newcastle/mainbanner}} =''yneA''= ==Aims== Today we aim to extract ''yneA''in the biobrick comapatible vector from yesterday's overnight culture. We aim to check the concentrat...) |
|||
| (24 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
| - | = | + | =Miniprep of filamentous cell part= |
==Aims== | ==Aims== | ||
| - | + | The aim of the experiment is to prepare stocks of the plasmid DNA containing our filamentous cell part. | |
| - | ==Materials and Protocol== | + | ==Materials and Protocol== |
| + | Please refer to protocols mentioned below for materials required: | ||
| + | *[[Team:Newcastle/Qiagen_Minipreps|Plasmid extraction protocol]] | ||
| + | *[[TeamNewcastleNanoDrop_Spectrophotometer|Nanodrop protocol]] | ||
==Results== | ==Results== | ||
| - | = | + | {|border=1 |
| + | |- | ||
| + | ! | ||
| + | !''yneA'' 1 | ||
| + | !''yneA'' 2 | ||
| + | !''yneA'' 3 | ||
| + | !''yneA'' 4 | ||
| + | |- | ||
| + | !Concentration of DNA ng/µl | ||
| + | !247.3 | ||
| + | !233.6 | ||
| + | !456.6 | ||
| + | !140.0 | ||
| + | |} | ||
| + | |||
| + | '''Table 1''': Nanodrop spectrophotometer results. Table represents the amount of plasmid present in µl/ml quantity. | ||
| + | |||
| + | [[Image:Nanodrop1882010.jpeg|350px]] | ||
| + | '''Figure 1''': Screenshot of ''yneA'' tube 1 | ||
| + | [[Image:Nanodrop18820102.jpeg|350px]] | ||
| + | '''Figure 2''': Screenshot of ''yneA'' tube 2 | ||
| + | [[Image:Nanodrop18820103.jpeg|350px]] | ||
| + | '''Figure 3''': Screenshot of ''yneA'' tube 3 | ||
| + | [[Image:Nanodrop18820104.jpeg|350px]] | ||
| + | '''Figure 4''': Screenshot of ''yneA'' tube 4 | ||
| + | |||
| + | ==Discussion== | ||
| + | The concentration of the different tubes range from 140.0 µl/ml to 456.6 µl/ml. The standard value for miniprep extraction is ~150 µg/ml. | ||
| + | |||
| + | ==Conclusion== | ||
| + | High DNA concentration was obtained. | ||
| + | |||
| + | '''Go back to our main [[Team:Newcastle/notebook| Lab book]] page''' | ||
| + | |||
| + | =Double digest of filamentous cell part, pSB1C3 and pGFP-rrnB= | ||
| + | We performed double digests of the filamentous cell part and pSB1C3 using EcoRI and SpeI, ready for ligation. | ||
| + | |||
| + | We also performed double digests of the filamentous cell part and pGFP-rrnB using EcoRI and NheI, ready for ligation. | ||
| + | |||
| + | ==Materials and Protocol== | ||
| + | |||
| + | Please refer to [[Team:Newcastle/Restriction_digests|Restriction digests]]. | ||
| - | |||
{{Team:Newcastle/footer}} | {{Team:Newcastle/footer}} | ||
Latest revision as of 00:05, 28 October 2010

| |||||||||||||
| |||||||||||||
Contents |
Miniprep of filamentous cell part
Aims
The aim of the experiment is to prepare stocks of the plasmid DNA containing our filamentous cell part.
Materials and Protocol
Please refer to protocols mentioned below for materials required:
Results
| yneA 1 | yneA 2 | yneA 3 | yneA 4 | |
|---|---|---|---|---|
| Concentration of DNA ng/µl | 247.3 | 233.6 | 456.6 | 140.0 |
Table 1: Nanodrop spectrophotometer results. Table represents the amount of plasmid present in µl/ml quantity.
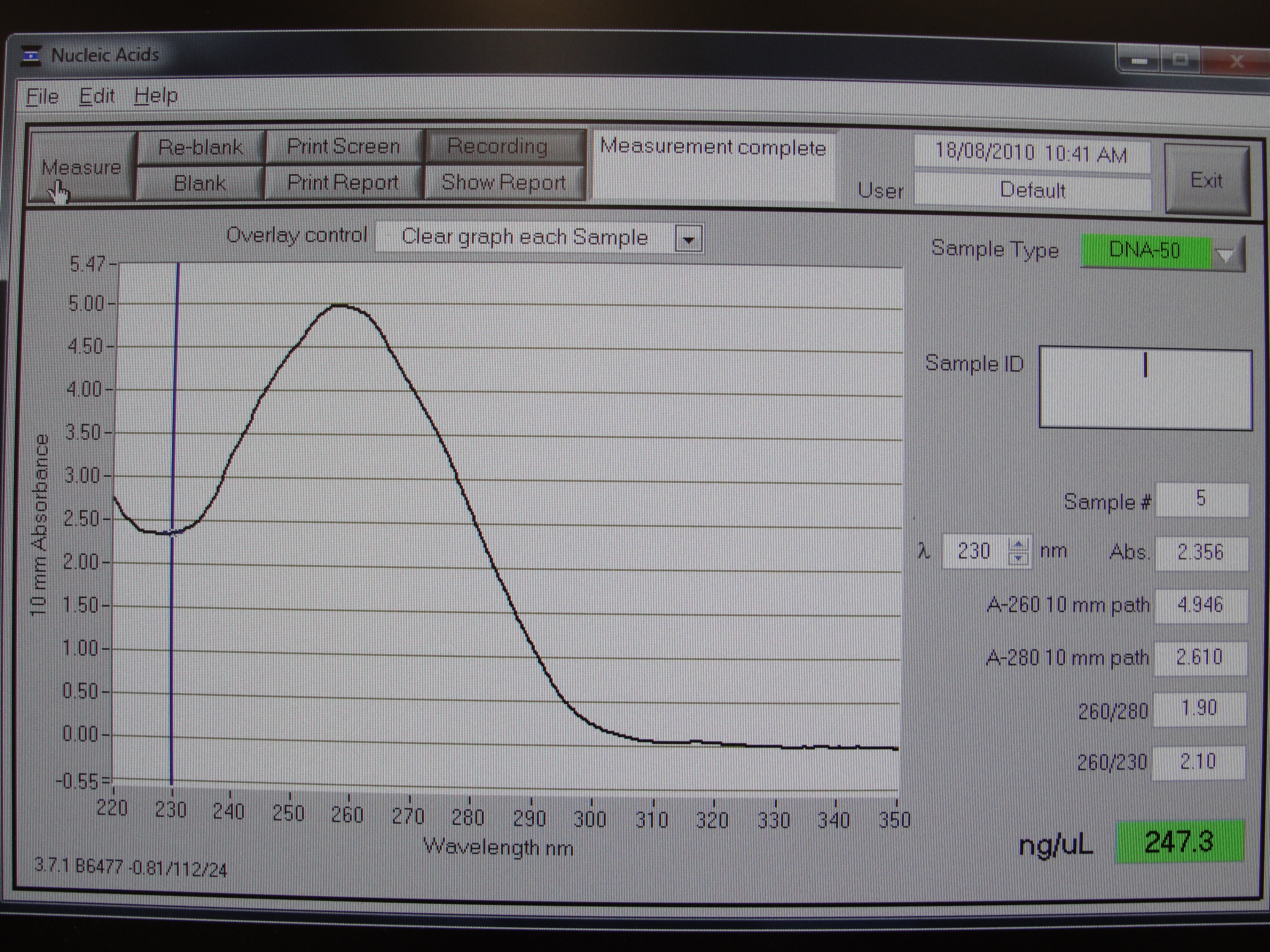 Figure 1: Screenshot of yneA tube 1
Figure 1: Screenshot of yneA tube 1
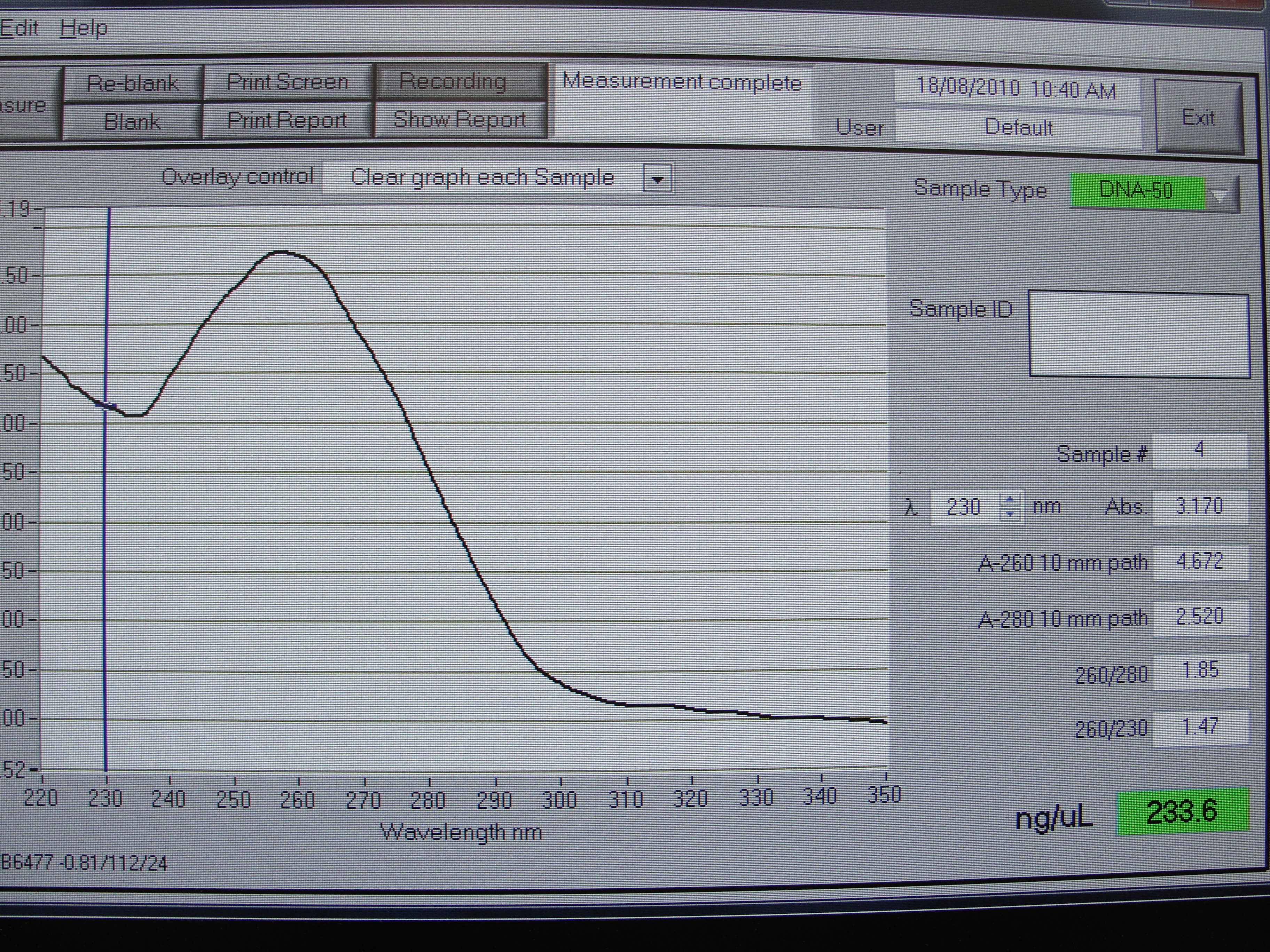 Figure 2: Screenshot of yneA tube 2
Figure 2: Screenshot of yneA tube 2
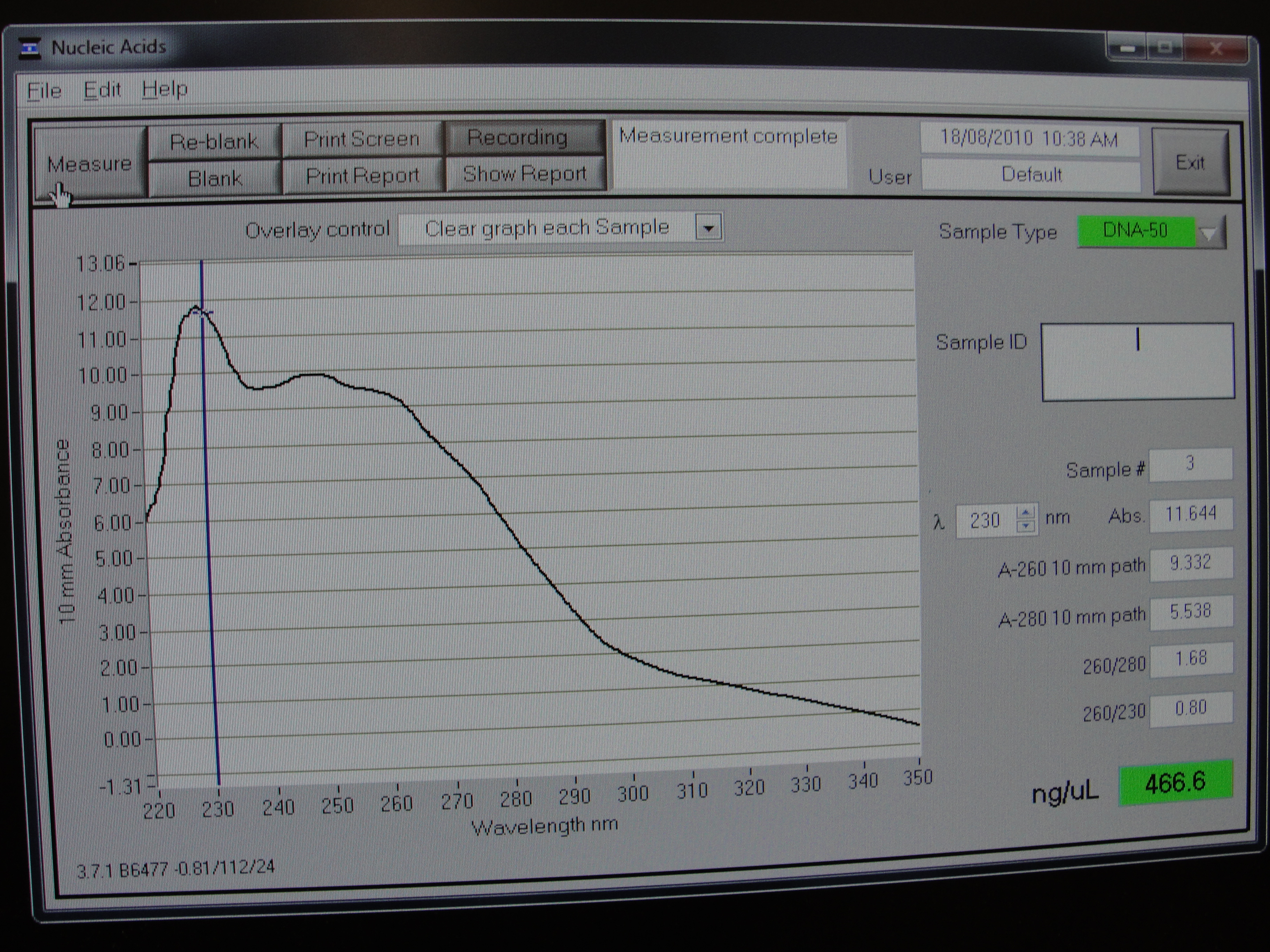 Figure 3: Screenshot of yneA tube 3
Figure 3: Screenshot of yneA tube 3
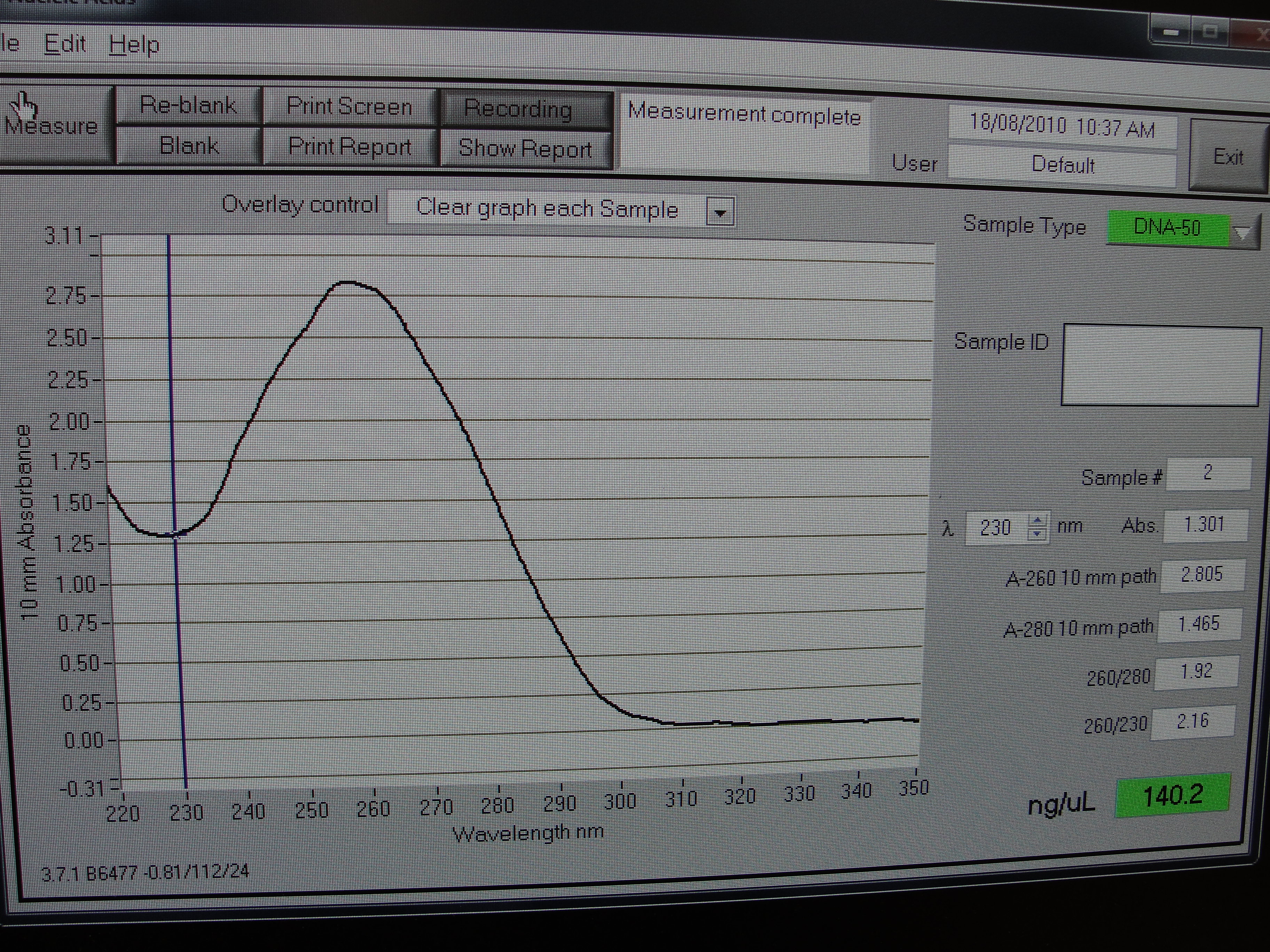 Figure 4: Screenshot of yneA tube 4
Figure 4: Screenshot of yneA tube 4
Discussion
The concentration of the different tubes range from 140.0 µl/ml to 456.6 µl/ml. The standard value for miniprep extraction is ~150 µg/ml.
Conclusion
High DNA concentration was obtained.
Go back to our main Lab book page
Double digest of filamentous cell part, pSB1C3 and pGFP-rrnB
We performed double digests of the filamentous cell part and pSB1C3 using EcoRI and SpeI, ready for ligation.
We also performed double digests of the filamentous cell part and pGFP-rrnB using EcoRI and NheI, ready for ligation.
Materials and Protocol
Please refer to Restriction digests.
 
|
 "
"