Team:Washington/Gram Positive/Build
From 2010.igem.org
(→Order Oligonucleotides) |
(→Generating Mutant DNA) |
||
| (98 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | __NOTOC__ | ||
{{Template:Team:Washington/Templates/Header}} | {{Template:Team:Washington/Templates/Header}} | ||
<html> | <html> | ||
| Line 24: | Line 25: | ||
</html> | </html> | ||
<!---------------------------------------PAGE CONTENT GOES BELOW THIS----------------------------------------> | <!---------------------------------------PAGE CONTENT GOES BELOW THIS----------------------------------------> | ||
| - | = | + | =Building Mutant CapD_CP= |
| + | To build the mutant proteins, we follow the path of the central dogma. First, we created DNA that contains our mutations. Second, we induced our transformed cells containing the desired DNA to express the mutant proteins. Lastly, we harvested the proteins by lysing open the cells and filtering out non-desired cell components. | ||
| - | == | + | ==Generating Mutant DNA== |
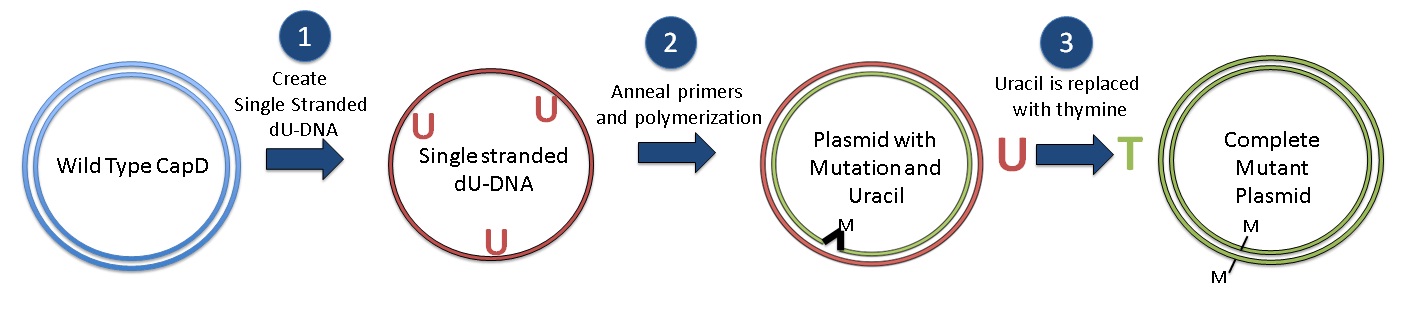
| - | [[Image: | + | [[Image:Washington_Kunkel_summary_revised2.jpg|center|thumb| 760px| Kunkel Mutagenesis Protocol: Generate single stranded dU-DNA, Anneal primers, polymerization, Synthesize Mutant Plasmids and replace uracil with thymine to complete]] |
| - | + | After we came up with the desired mutant protein designs, we employed the [[Team:Washington/Protocols/KunkelCapD|Kunkel Mutagenesis]] method to generate the desired mutant DNA. Kunkel mutagenesis is a three step process. First, we obtained ssDNA of wild-type CapD_CP gene from transformed cells that contain the CapD_CP gene and lack the enzyme to destroy uracil. The presence of uracil is used later to obtain the correct DNA strand. The second step involved annealing our mutation-containing primers to the ssDNA and polymerizing the strand that contains our desired mutations. The result is a double-stranded DNA, consisting of a wild-type CapD_CP strand and a mutations-containing (desired) strand. Lastly, to obtain a dsDNA that consists of only the desired strands, we transformed it into another type of cell that contains enzymes to destroy the uracil-containing strand. Once the uracil-containing wild-type strand is destroyed, the complementary strand is synthesized, resulting in a dsDNA that contains only our desired mutations.[[#References | [1]]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[ | + | |
| + | ==Protein Expression and Purification== | ||
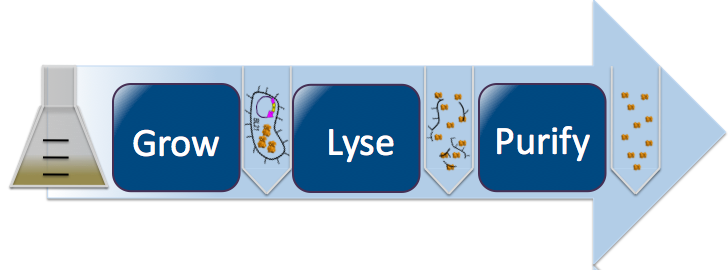
| + | [[Image:Washington_lyse_revised4.png|thumbnail|700px|left|Protein Purification Process]] | ||
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | <br /> | ||
| + | Once we obtained cells transformed with our desired mutant DNA, we induced the cells to express the mutant protein. By introducing Isopropyl β-D-1-thiogalactopyranoside (IPTG), an allolactose mimic, we induce E. Coli to produce our protein. IPTG binds with the lac inhibitor protein and activates the lac operon, turning on the CapD_CP gene and causing production of our mutant protein. For this step, we used two different protocols: [https://2010.igem.org/Team:Washington/Protocols/50mLPurificationCapD small scale] and [https://2010.igem.org/Team:Washington/Protocols/1LPurificationCapD large scale]. The concepts described below are the same for both protocols. To harvest our mutant proteins, we needed to first lyse open the induced cells and then purified out our proteins (refer to small/large scale protocol). For the purification procedure, we employed Talon beads. CapD_CP's designed histidine tags bind to the beads, whilst everything else flows through. The result of this process is our purified mutated CapD_CP proteins. | ||
| + | ==References== | ||
| + | 1. T.A. KUNKEL, P NATL ACAD SCI USA 82, 488 (JANUARY 1985, 1985) | ||
<!---------------------------------------PAGE CONTENT GOES ABOVE THIS----------------------------------------> | <!---------------------------------------PAGE CONTENT GOES ABOVE THIS----------------------------------------> | ||
<div style="text-align:center"> | <div style="text-align:center"> | ||
| - | '''← [[Team:Washington/ | + | '''← [[Team:Washington/Gram Positive/Design|Designing the Gram(+) Therapeutic]]''' |
| | ||
| | ||
| | ||
| - | '''[[Team:Washington/ | + | '''[[Team:Washington/Gram Positive/Test|Testing the Gram(+) Therapeutic]] →''' |
</div> | </div> | ||
{{Template:Team:Washington/Templates/Footer}} | {{Template:Team:Washington/Templates/Footer}} | ||
Latest revision as of 20:37, 27 October 2010
Building Mutant CapD_CP
To build the mutant proteins, we follow the path of the central dogma. First, we created DNA that contains our mutations. Second, we induced our transformed cells containing the desired DNA to express the mutant proteins. Lastly, we harvested the proteins by lysing open the cells and filtering out non-desired cell components.
Generating Mutant DNA
After we came up with the desired mutant protein designs, we employed the Kunkel Mutagenesis method to generate the desired mutant DNA. Kunkel mutagenesis is a three step process. First, we obtained ssDNA of wild-type CapD_CP gene from transformed cells that contain the CapD_CP gene and lack the enzyme to destroy uracil. The presence of uracil is used later to obtain the correct DNA strand. The second step involved annealing our mutation-containing primers to the ssDNA and polymerizing the strand that contains our desired mutations. The result is a double-stranded DNA, consisting of a wild-type CapD_CP strand and a mutations-containing (desired) strand. Lastly, to obtain a dsDNA that consists of only the desired strands, we transformed it into another type of cell that contains enzymes to destroy the uracil-containing strand. Once the uracil-containing wild-type strand is destroyed, the complementary strand is synthesized, resulting in a dsDNA that contains only our desired mutations. [1]
Protein Expression and Purification
Once we obtained cells transformed with our desired mutant DNA, we induced the cells to express the mutant protein. By introducing Isopropyl β-D-1-thiogalactopyranoside (IPTG), an allolactose mimic, we induce E. Coli to produce our protein. IPTG binds with the lac inhibitor protein and activates the lac operon, turning on the CapD_CP gene and causing production of our mutant protein. For this step, we used two different protocols: small scale and large scale. The concepts described below are the same for both protocols. To harvest our mutant proteins, we needed to first lyse open the induced cells and then purified out our proteins (refer to small/large scale protocol). For the purification procedure, we employed Talon beads. CapD_CP's designed histidine tags bind to the beads, whilst everything else flows through. The result of this process is our purified mutated CapD_CP proteins.
References
1. T.A. KUNKEL, P NATL ACAD SCI USA 82, 488 (JANUARY 1985, 1985)
 "
"