Team:Tokyo Tech/Project/Artificial Cooperation System/lux act rep/Pluxact/assay1
From 2010.igem.org
Contents |
luxR activation promoter (BBa_R0062) assay
In case of presence/absence of 3OC6HSL
Abstract
We characterized the strength of this promoter which has already been done before in BioBrick in order to design a new promoter based on this data. First, we measured fluorescence by flow cytometry 3 hour after addition of 100nM 3OC6HSL to confirm R0062, which is an existing BioBrick promoter activated by LuxR/3OC6HSL complex.
Introduction
We characterized the strength of this promoter which has never been done before in BioBrick in order to design new promoter base on this data. In the Artificial Cooperation System, we inserted chloramphenicol resistance coding sequence to this promoter. Thus, this promoter plays an important role in Artificial Cooperation System.
Results
The result is shown in fig.○. The expression of GFP with 3OC6HSL around 30 holds increased comparing with the expression without 3OC6HSL.
Conclusion
We confirmed that 3OC6HSL activated luxR activation promoter, as expected.
Materials & Methods
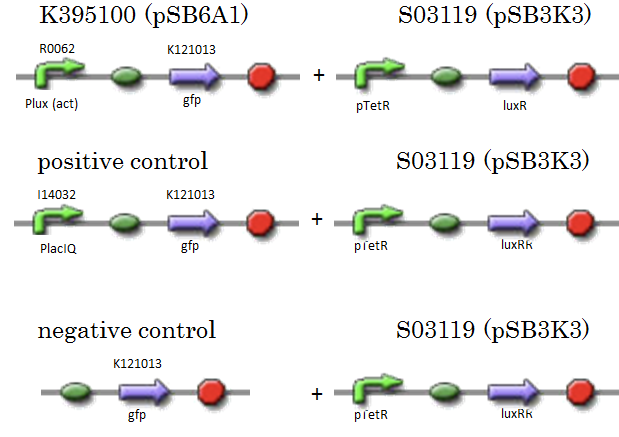
We constructed K395100 combining R0062 and K121013. K121013 is a promoter-less gfp reporter (rbs-gfp-ter-ter) on pSB6A1. S03119 is a LuxR generator which is regulated by PTetR, which is repressed by TetR. In this experiment, we don’t use TetR, so S03119 functions as a LuxR constitutive generator. The backbone of S03119 is pSB1A2, which is a high copy plasmid, so we changed the backbone from pSB1A2 to pSB3K3. We used a fusion of PlacIq (I14032) to gfp (K121013) as a positive control and used promoterless gfp (K121013) as a negative control.
- samples
- [Plux act - GFP](BBa_K395100) on pSB6A1 + [PtetR – LuxR] on pSB3K3
- positive control: [PlacIq(constitutive promoter) - GFP] on pSB6A1+ [PtetR – LuxR] on pSB3K3
- negative control:. [promoterless - GFP] on pSB6A1+ [PtetR – LuxR] on pSB3K3
- Strain
DH5&alpha
- protocol
- Prepare overnight culture.
- Take 30 ul of the overnight culture into LB + antibiotics (Amp + Kan). (→fresh culture)
- Incubate the fresh culture until the observed O.D. reaches around 0.60.
- Each sample was divided into 2. Prepare and add 3OC6HSL mixture to one, and add DMSO mixture to the other. The final concentration of 3OC6HSL is 100nM.
- Induction for 3 hours at 37°C.
- Fluorometer (FLA5200) and flow cytometry measurements for GFP expression.
 "
"