Team:Tokyo Tech/Project/Apple Reporter2
From 2010.igem.org
(→Materials and Methods) |
(→Materials and Methods) |
||
| Line 70: | Line 70: | ||
'''Strains of ''E. coli''''' | '''Strains of ''E. coli''''' | ||
<Br>''E. coli'' BL21 (DE3) | <Br>''E. coli'' BL21 (DE3) | ||
| + | |||
'''Varieties of plasmid'''<Br> | '''Varieties of plasmid'''<Br> | ||
MpAAT1 on pSB6A1<Br> | MpAAT1 on pSB6A1<Br> | ||
Trx on pACYC184 | Trx on pACYC184 | ||
| + | |||
'''Substrate'''<Br> | '''Substrate'''<Br> | ||
| Line 83: | Line 85: | ||
100 mM IPTG (final 100 μM)<Br> | 100 mM IPTG (final 100 μM)<Br> | ||
20% arabinose (final 0.1%)<Br> | 20% arabinose (final 0.1%)<Br> | ||
| + | |||
'''Solution'''<Br> | '''Solution'''<Br> | ||
| Line 92: | Line 95: | ||
This artificial gene was ligated with vector pSB6A1 as MpAAT1 expression plasmid.<Br> | This artificial gene was ligated with vector pSB6A1 as MpAAT1 expression plasmid.<Br> | ||
Moreover, we introduced pTrx6 into this expression plasmid to stabilize the ''MpAAT1'' gene product.<Br> | Moreover, we introduced pTrx6 into this expression plasmid to stabilize the ''MpAAT1'' gene product.<Br> | ||
| + | |||
'''MpAAT1 over expression conditions'''<Br> | '''MpAAT1 over expression conditions'''<Br> | ||
| Line 98: | Line 102: | ||
Therefore we utilized ''E. coli'' BL21 DE3 which has T7 RNA polymerase.<Br> | Therefore we utilized ''E. coli'' BL21 DE3 which has T7 RNA polymerase.<Br> | ||
Furthermore, arabinose was added in culture to induce Trx which has arabinose-induced promoter.<Br> | Furthermore, arabinose was added in culture to induce Trx which has arabinose-induced promoter.<Br> | ||
| + | |||
'''''E. coli'' BL21 DE3''' <Br> | '''''E. coli'' BL21 DE3''' <Br> | ||
| Line 116: | Line 121: | ||
→derive oil layer from supernatant solution with liquid-liquid extraction<Br> | →derive oil layer from supernatant solution with liquid-liquid extraction<Br> | ||
→Gas Chromatography analysis.<Br> | →Gas Chromatography analysis.<Br> | ||
| + | |||
'''Gas Chromatography analysis'''<Br> | '''Gas Chromatography analysis'''<Br> | ||
Revision as of 03:16, 28 October 2010
2-2 Fragrance
Contents |
Abstract
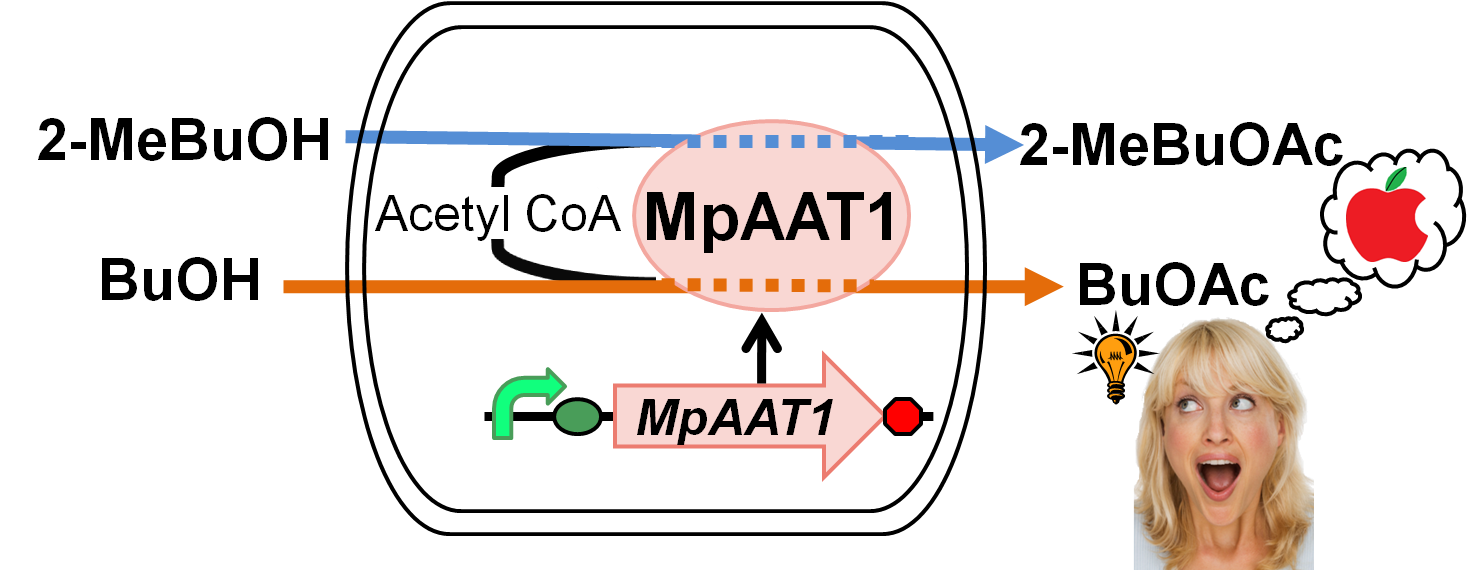
We designed apple fragrance expression device with MpAAT1[1]. MpAAT1 is able to produce ester compounds with apple fragrance using alcohols and Acetyl-CoA. Fig. 2-2-1 shows the outline of the device. We performed gas chromatography to confirm the product of esters. The results revealed that MpAAT1 could produce 2-methylbutyl acetate and butyl acetate using 2-methyl butanol and butanol respectively (Fig. 2-2-2). 2-methylbutyl acetate and butyl acetate are known as the Apple fragrance.
Introduction
MpAAT1 gene was isolated from Malus pumila (popular apple)[2]. This gene is expressed in leaves, flowers and fruit of apple. The recombinant enzyme (MpAAT1) to produce esters involved in apple fragrance, utilize various alcohol as substrates such as straight chain (C3–C10), branched chain, aromatic and terpene alcohols. In addition, various kinds of CoA derivatives, such as acetyl-CoA, are used for producing esters by MpAAT1. Both alcohol and CoA derivative are required for successful ester synthesis. The major components involved in apple fragrance are butyl acetate and 2-methylbutyl acetate[3]. We engineered apple fragrance expression device to produce these molecules by MpAAT1. This device is used as a reporter in Artificial Cooperation System of our projects, when dying cells are recued by its counterpart, it gives an apple fragrance for a token of their gratitude.
Result
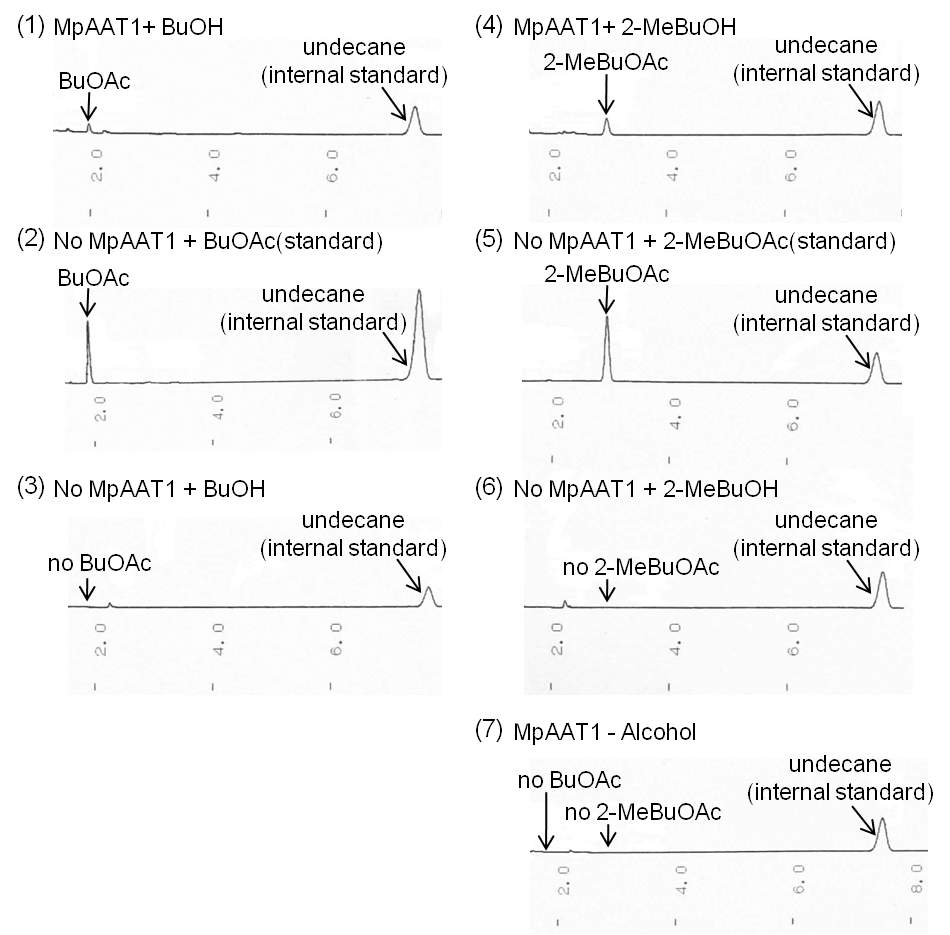
We transformed MpAAT1([http://partsregistry.org/Part:BBa_K395602 BBa_K395602]) on pSB6A1 along with pTrx6 into E.coli BL21 DE3, and cultured after addition of alcohols(2-MeBuOH or BuOH) as substrates. After 12 hours of incubation, we extracted organic solution layer from the culture and analyzed by gas chromatography (Fig. 2-2-2). Peaks of the esters producing apple fragrance (2-MeBuOAc or BuOAc) were detected.
By comparing (1) with (2), BuOAc was confirmed when E. coli has MpAAT1. Moreover, by Comparing (1) with (3), BuOH (substrate) doesn’t contain BuOAc as impurity. By the same token, 2-MeBuOAc was confirmed when E. coli has MpAAT1 by comparing (4) with (5). Moreover, by Comparing (4) with (6), 2-MeBuOH (substrate) doesn’t contain 2-MeBuOAc as impurity. (7) shows E. coli which has MpAAT1 is able to convert alcohols (substrates) into fragrance molecules grown in no substrate culture. From these result, E. coli which has MpAAT1 successfully was able to produce 2-methylbutyl acetate and butyl acetate using 2-methyl butanol and butanol respectively.
Discussion
From the result of the experiment above, we can conclude that apple fragrance expression device was able to produce esters using alcohols added as substrates. Moreover, we synthesized MpAAT1 and esters using E.coli BL21 DE3 as a chassis, which was reported to be implausible in former report(s) [1]. In 2008, C.R. Shen and J.C. Liao[4] succeeded in synthesizing butanol from E.coli. If we take advantage of this engineered E.coli, we could produce apple fragrance ester without the addition of substrate.
Materials and Methods
Strains of E. coli
E. coli BL21 (DE3)
Varieties of plasmid
MpAAT1 on pSB6A1
Trx on pACYC184
Substrate
Butanol (final 0.4%)
2-methyl butanol (final 0.2%)
Inducer
100 mM IPTG (final 100 μM)
20% arabinose (final 0.1%)
Solution
Undecane solution: undecane 10 μL + ether 990 μL
MpAAT1 expression construct.
The coding sequence of MpAAT1 gene was synthesized and optimized sequence by Mr.Gene.
This artificial gene was ligated with vector pSB6A1 as MpAAT1 expression plasmid.
Moreover, we introduced pTrx6 into this expression plasmid to stabilize the MpAAT1 gene product.
MpAAT1 over expression conditions
Artificial gene has T7 promoter on the upstream of MpAAT1.
This promoter works by taking over T7 RNA polymerase from E. coli.
Therefore we utilized E. coli BL21 DE3 which has T7 RNA polymerase.
Furthermore, arabinose was added in culture to induce Trx which has arabinose-induced promoter.
E. coli BL21 DE3
E. coli BL21 DE3 express T7 RNA polymerase by IPTG induction.
Therefore we added 3 μL of 100 mM IPTG and 15 μL of 20% arabinose in LB culture in order to express MpAAT1 and Trx in E. coli BL21 DE3.
Expression of MpAAT1 recombinant protein in E. coli
O/N E. coli
→100-fold dilution
→2 hour fresh culture
→make up the OD590 = 0.1 with LB culture
→add substrate,antibiotics and inducer
→O/N
→harvest (7,000×g, 3 min)
→collect supernatant solution
→separating liquid layer and oil layer with ether (shake supernatant solution 0.5 mL, ether 0.5 mL and undecane soln. 2 μL)
→derive oil layer from supernatant solution with liquid-liquid extraction
→Gas Chromatography analysis.
Gas Chromatography analysis
Gas Chromatography : SHIMADZU GAS CHROMATOGRAPH GC-14B
Column: J&W SCIENTIFIC, DB-17, Film thickness 0.25 μm, Column Dimensions 15 m × 0.320 mm, Temperature Limits 40°C to 280°C (300°C Program)
Conditions: column temperature 35°C, injector temperature 180°C, detector temperature 180°C
Sample was injected 5 μL.
reference
[1] Edwige J. F. Souleyre, FEBS Journal, 272, 3132–3144 (2005)
[2] Young H, J Sci Food Agric, 71, 329-336 (1996)
[3] [http://www.ncbi.nlm.nih.gov/nucleotide/52139952 GenBank accession number AY707098]
[4] C.R. Shen, Metabolic Engineering, 10, 312–320 (2008)
 "
"