Team:Newcastle/26 August 2010
From 2010.igem.org
(Difference between revisions)
(New page: =''yneA''= ==PCR (Repeat)== ===Aim=== To repeat the PCR that we did yesterday using the correct ''rocF'' primers. ===Materials and Protocol=== Please...) |
(→Results) |
||
| (31 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | {{Team:Newcastle/mainbanner}} | |
| - | |||
| - | |||
| - | + | =Screening ''Bacillius subtilis'' transformants= | |
| - | == | + | ==Aim== |
| + | The aim of the experiment is to identify those colones that have the plasmid integrated at the correct position in the chromosome, which is the ''amyE'' locus. Those that have integrated at the correct position will not be able to break down starch, which can be tested by exposing the colonies on the starch plates to iodine. | ||
| - | + | ===Results=== | |
| + | Some of our colonies did not have halos, therefore the transformation and integration was successful for these colonies. | ||
| - | + | Below you can see cells from these positive colonies under the microscope. | |
| - | + | {| | |
| + | |- | ||
| + | |[[Image:Starchplate.jpg|thumb|250px|centre]] | ||
| + | | | ||
| + | |[[Image:Starchplate2.jpg|thumb|250px|centre]] | ||
| + | |} | ||
| + | {| | ||
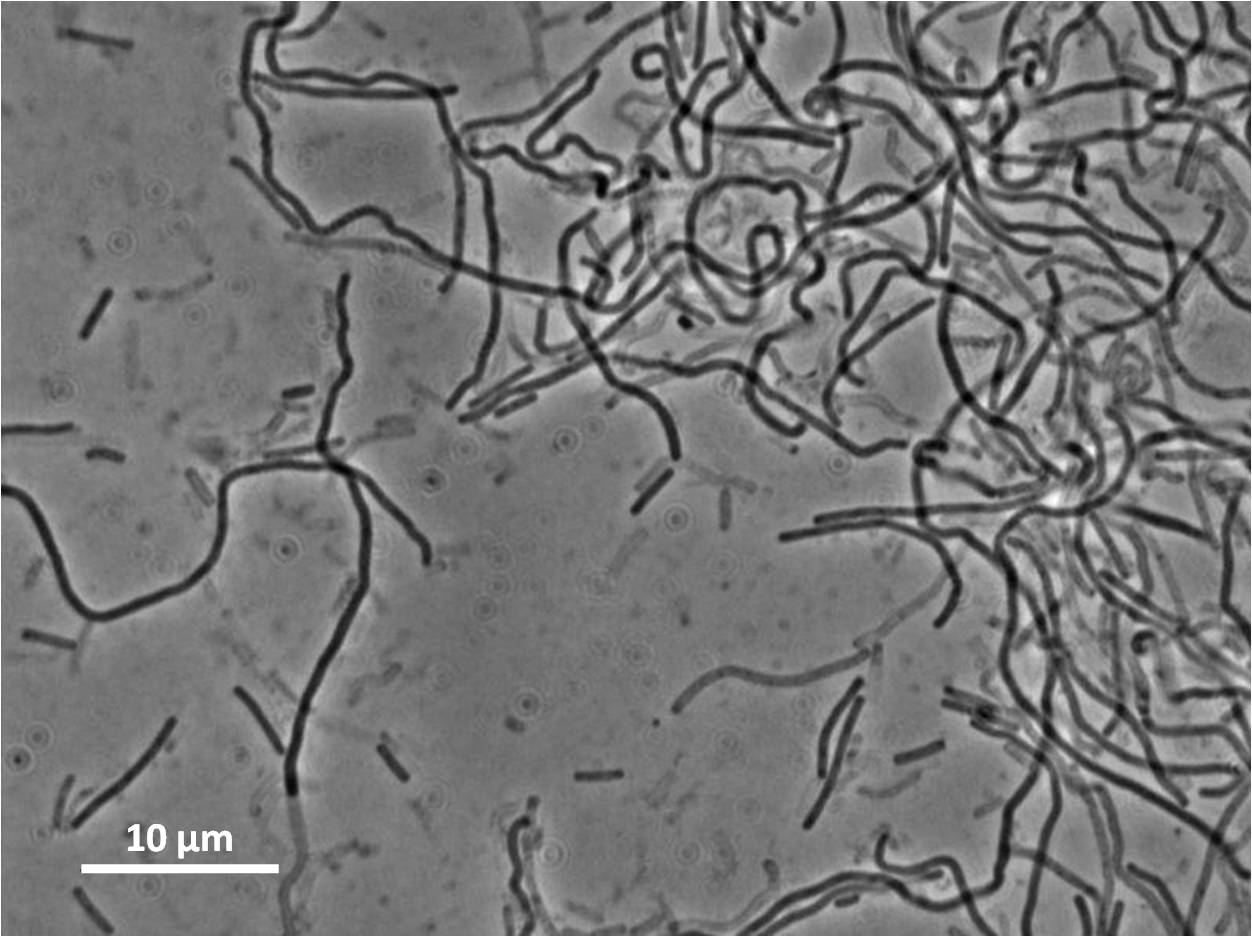
| + | |[[Image:Newcastle_filamentous_pc_expt1.jpg|thumb|Filamentous cells|250px|centre]] | ||
| + | | | ||
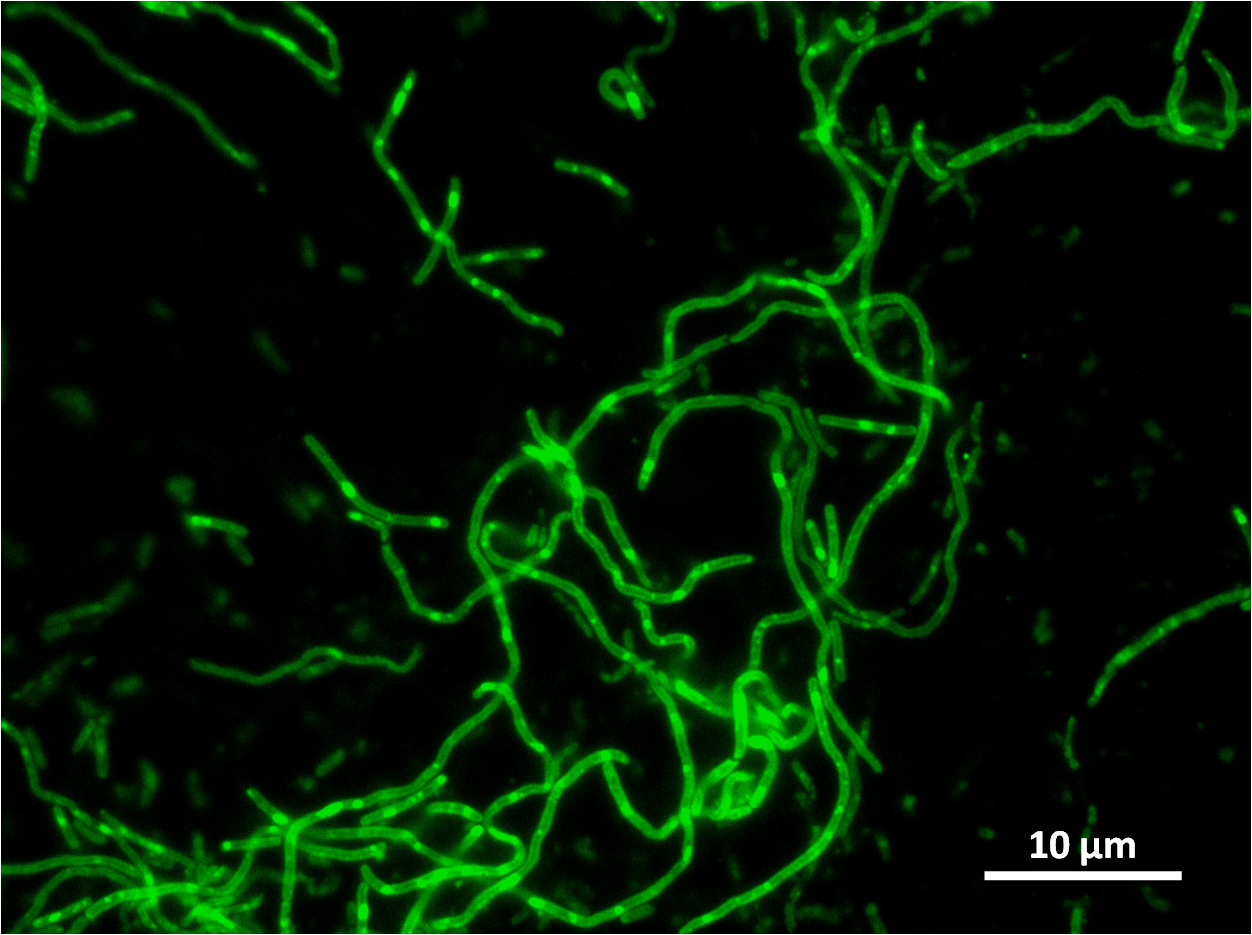
| + | |[[Image:Newcastle_filamentous_gfp_expt1.jpg|thumb|Filamentous cells showing GFP signal |250px|centre]] | ||
| + | | | ||
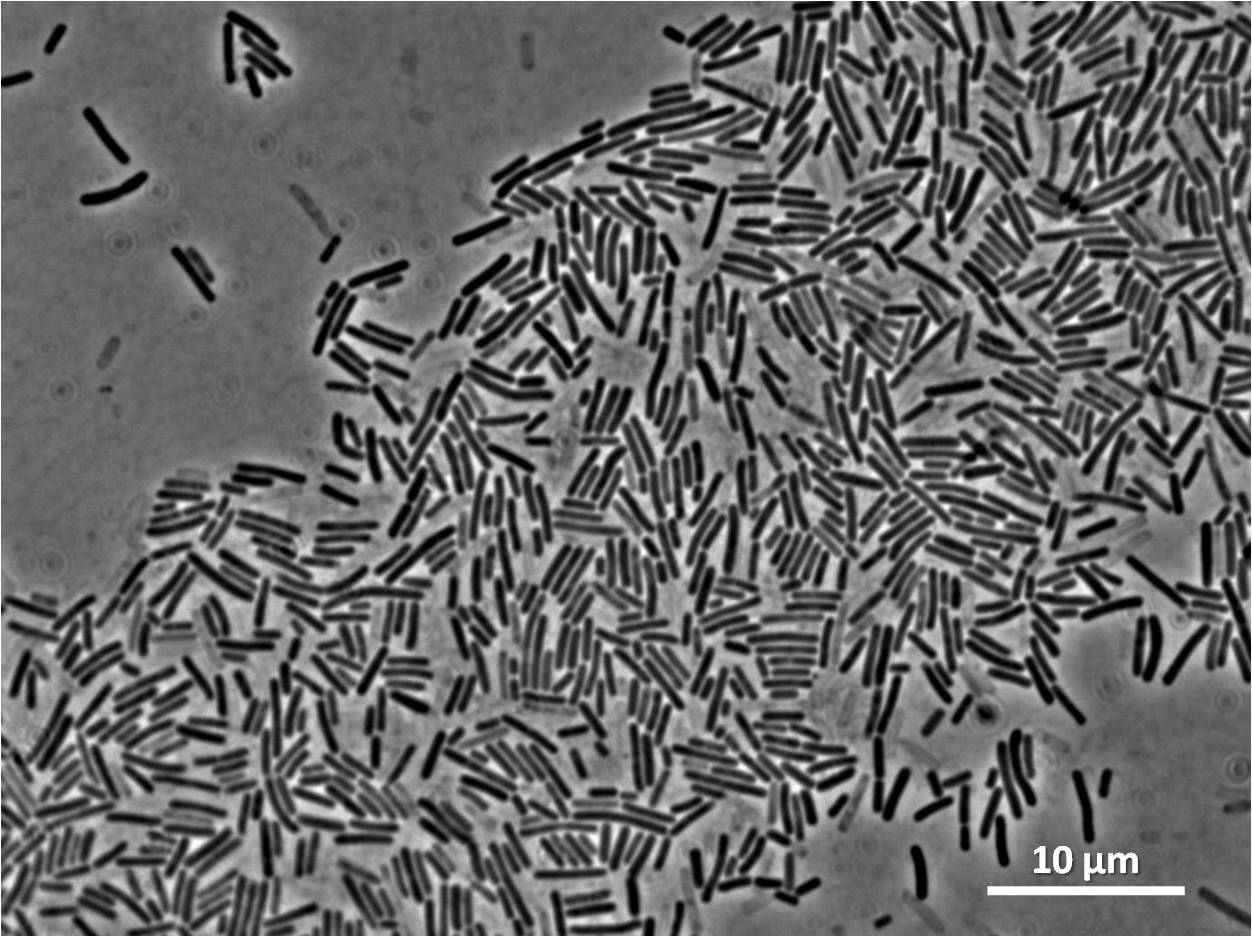
| + | |[[Image:Newcastle_filamentous_control_pc_expt1.jpg|thumb|Normal ''Bacillus subtilis ''168|250px|centre]] | ||
| + | |} | ||
| - | |||
| - | |||
| - | |||
| - | + | {{Team:Newcastle/footer}} | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Latest revision as of 01:15, 28 October 2010

| |||||||||||||
| |||||||||||||
Screening Bacillius subtilis transformants
Aim
The aim of the experiment is to identify those colones that have the plasmid integrated at the correct position in the chromosome, which is the amyE locus. Those that have integrated at the correct position will not be able to break down starch, which can be tested by exposing the colonies on the starch plates to iodine.
Results
Some of our colonies did not have halos, therefore the transformation and integration was successful for these colonies.
Below you can see cells from these positive colonies under the microscope.
 
|
 "
"