Team:Newcastle/11 August 2010
From 2010.igem.org
(→Gibson cloning of the rocF BioBrick) |
(→Transformation of E. coli DH5α cells with ligated rocF fragments) |
||
| Line 3: | Line 3: | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
=Subtilin Immunity BioBrick= | =Subtilin Immunity BioBrick= | ||
Revision as of 00:04, 26 October 2010

| |||||||||||||
| |||||||||||||
Contents |
Subtilin Immunity BioBrick
Aims
Our aims for today are to run the gel electrophoresis to check whether we have the correct fragment sizes on the four parts that are we amplified yesterday. If the PCR worked, we will then perform gel extraction and then perform another gel electrophoresis for the extracted gel in order to obtain our BioBrick parts.
Materials and protocol
Please refer to the gel electrophoresis and the gel extraction protocols.
Results
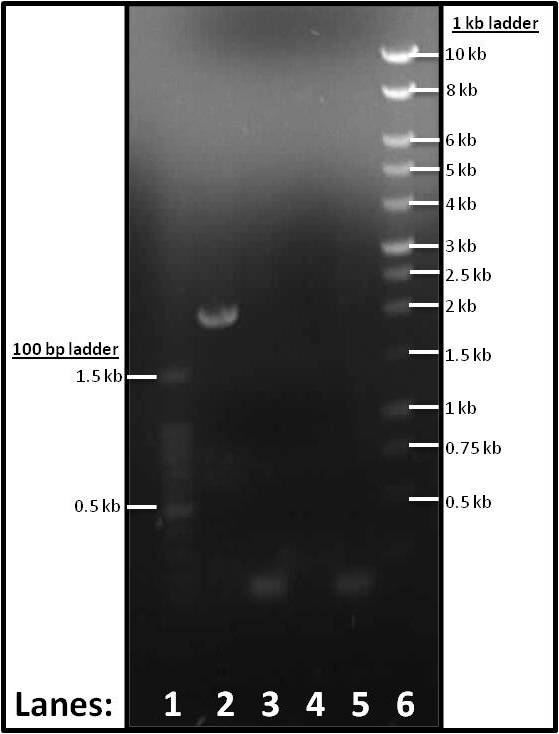
Figure 1: Gel electrophoresis of the PCR products of the parts required for the subtilin immunity BioBrick.
- Lane 1: 1 kb DNA ladder
- Lane 2: Plasmid Vector (pSB1C3)
- Lane 3: Promoter and RBS (pVeg-SpoVG)
- Lane 4: spaIFEG Gene Cluster
- Lane 5: Double terminator
- Lane 6: 100 bp DNA ladder
Discussion
Plasmid Vector (lane 2), Promoter & RBS (lane 3) and Double terminator (lane 5) showed up. spaIFEG PCR tube (lane 4) did not show up. We think that this occurrence was due to the Tm error (it should be 63°C as opposed to 46°C). Therefore, another PCR and gel electrophoresis were performed. Please see tomorrow's lab book page for this.
Go back to our main Lab book page
 
|
 "
"