Team:SDU-Denmark/project-p
From 2010.igem.org
Contents |
Our Parts
<groupparts>iGEM010 SDU-Denmark</groupparts>
Characterization of parts
K343004
The FlhDC operon is the master regulator of flagella synthesis. A more detailed description of the operon can be found here
In our system the purpose of the composite part is to hyper flagellate our cells so that a grater force can be generated in the microtubes. The FlhDC operon is naturally found in the E. coli strain MG1655 genome. We extracted the operon and inserted a silent mutation (T to C) at position 822 in the operon because without the mutation this site is a Pst1 digestion site and it would therefor constitute problems when assembling the composit part.
We have made three FlhDC parts:
[http://partsregistry.org/Part:BBa_K343100 K343100] is the coding sequence of the native FlhDC operon with the Pst1 digestion site
[http://partsregistry.org/Part:BBa_K343000 K343000] is the coding sequence of the mutated FlhDC operon
[http://partsregistry.org/Part:BBa_K343004 K343004] is the composite part containing the TetR repressable promoter (constitutive when no TetR is pressent) + RBS (J13002), the K343000 part and the double terminator (B0015).
The composite part is caracterized firstly by using a motility assay and secondly by measuring plasmid stability and cell growth.
Motility assay
1. Motility assay (Experiment 1 with WT and negative control):
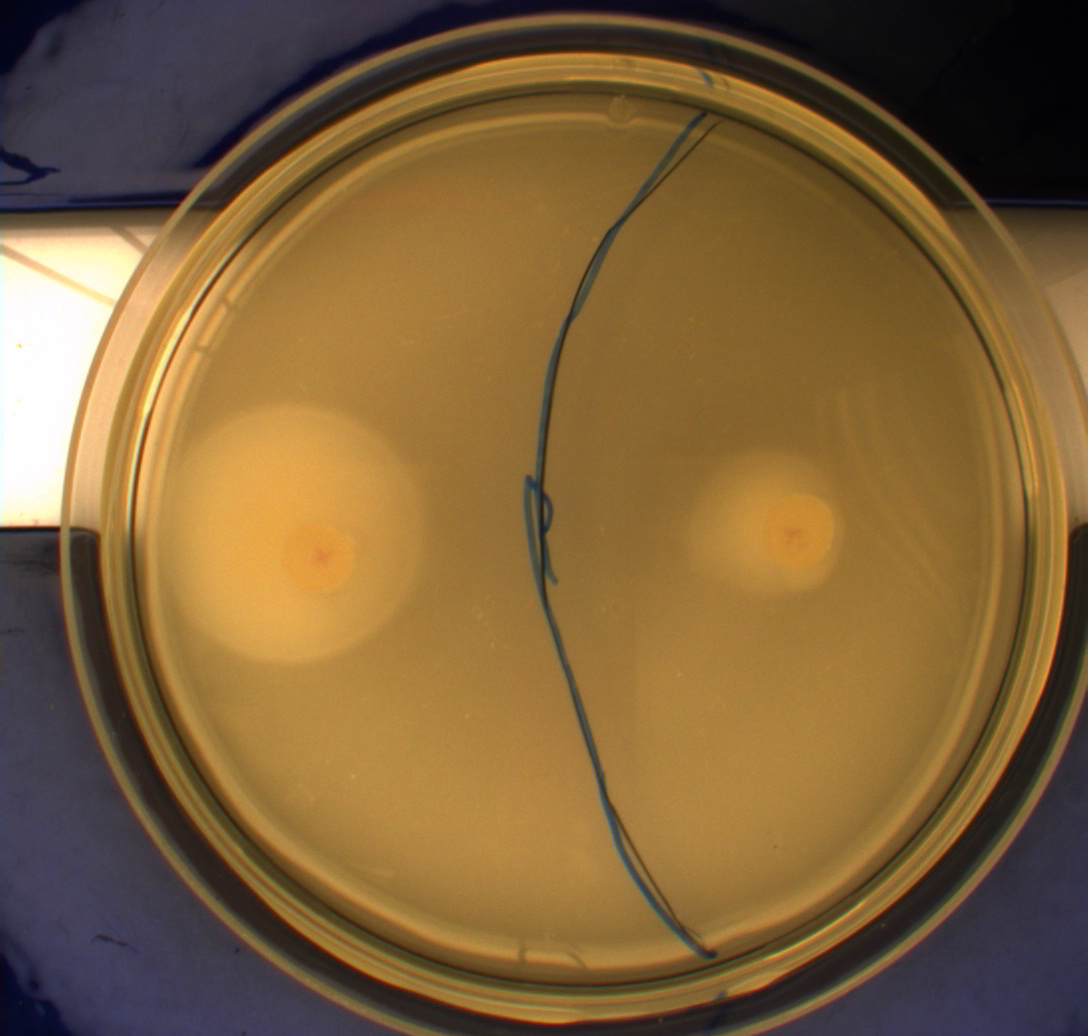
Growth of bacterial culture on semi-solid agar plates
The purpose of this experiment is to see if the cells containing pSB1C3-K343004 move farther than the wild type (MG1655) and the negative control (DH5alpha). This would be an indication of hyperflagellation. We also want to test if it makes a difference in the motility wether the bacteria contain low-medium- or high-copy plasmids.
For the motility experiments we added 5ul of an ON culture to petridishes containing motility agar (LB media with 0.3% agar) instead of regular LA (Luria agar). This semi-solid media lets the bacteria swim more easily.
The plates were incubated at 37 degrees celcius for 24 hours.



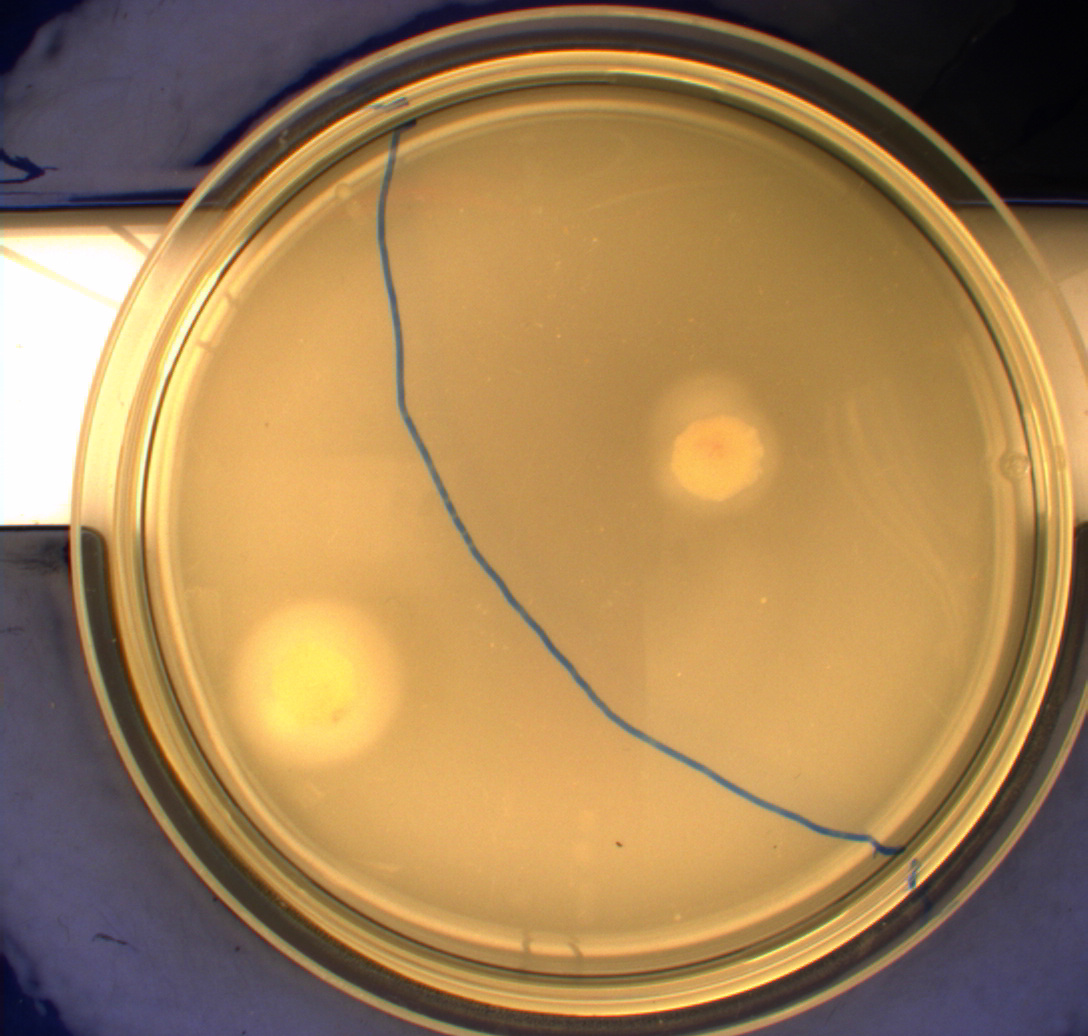
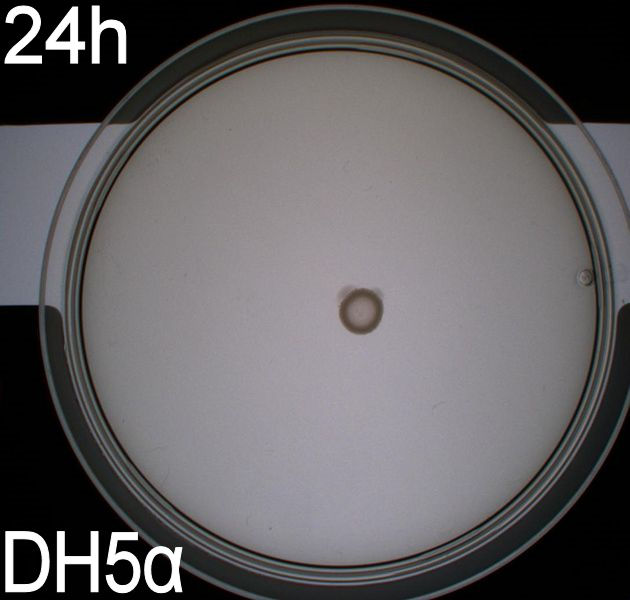
The upper two plates did not contain antibiotics, and therefore contamination colonies are seen.
The upper left picture is of E. coli strain DH5alpha that does not express flagella and therefor movement in the media should, as seen on the picture, be minimal. The upper right picture is of the wild type E. coli strain MG1655, this strain has about 8-10 flagella per cell. These cells are, as seen, expected to move farther than the DH5alpha but not as far as the transformed cells. The lower left picture is of E. Coli strain MG1655 with pSB1C3-K343004 this shows that these bacteria move farther than the wild type and the negative control. The lover right picture is of E. coli strain MG1655 with pSB3K3-K343004 these bacteria move farther than the wild type, the negative control and MG1655 with pSB3K3-K343004.
From the pictures above we can definately se that the bacteria containing our part is much more motile than the wild type. We assume this is caused by overexpression of the FlhDC master flagella operon which leads to hyperflagellation of the cells.
The two buttom pictures show that bacteria with pSB1C3-K343004 have not moved as far as the bacteria containing pSB3K3-K343004. pSB1C3 is a high copy plasmid while pSB3K3 is a low-medium copy plasmid. The promoters in K343004 is a constitutive promoter (tetR repressable promoter). Bacteria containing a high copy plasmid with a constitutive promoter are more metabolically challanged than bacteria containing a low- or medium-copy plasmid with a constitutive promoter because of the higher number of plasmids per the cell. Therefore the high copy plasmid bacteria are less motile than low- or medium-copy plasmid bacteria.
2. Motility assay (doublicate with WT, negative- and positive-control)after 24 hours:
Growth of bacterial culture on semi-solid agar plates


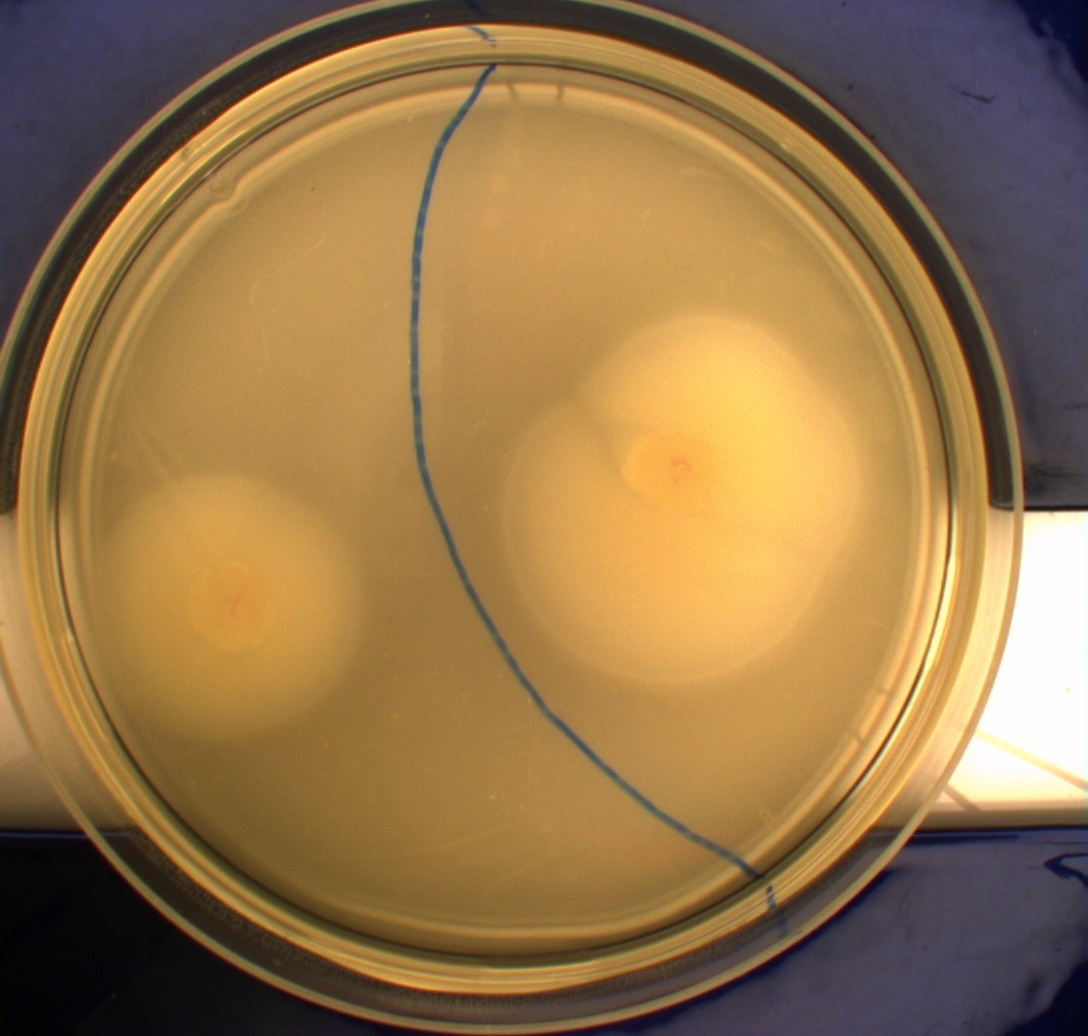
The duplicate of the motility assay shows:
- very little motility of the non-flagellated negative control DH5alpha. Less than in the first assay
- Minimal motility of the wild type MG1655, though they have colonized a bigger area on the plate than DH5aplha. The MG1655 cells also show less motility in this assay than in the first. Also the colony morphorlogy is wvery different in the two assays
The only difference between the two assays is that the second assay was plated out and grown on plates in a class II lab because of the positive control.
- Greate motility of the hyper flagellated E. coli strain H10407 (class II pathogen) positive control
- MG1655 cells with pSB1C3-K343004 have moved farther than both the negative control and the wild type, but not as far as the positive control. On both assays of this transformant a "line" is visable arround the edge of the colony, we think this is an indication that these cells are not moving much further. This transformant show similar motility as in the first assay
- MG1655 cells with pSB3K3-K343004 have moved farthest of all 5 cultures. These cells and the positive control both look as though they are still swimming. This transformant show similar motility as in the first assay
The plates were left in the 37 degrees incubator for another 24 hours to see if our assumptions are correct.
3. Motility assay (doublicate with WT, negative- and positive-control)after 48 hours:
4. Stability assay:
5. Growth measurement:
The purpose of this assay is to see if our transformants deviate from the wild type in growth rate. In the growth measurement assay we have measured OD at 550 nm every hour for 12 hours and at hour 24. In the experimental setup we used we see no lag phase in any of the measurments.
The graph below shows the growth of our wild type E. coli strain MG1655, the MG1655 transformed with K343004 in pSB3K3 and in pSB1C3.

From our data we see no significant difference between the transformed bacteria and the wild type.
K343007
The part K343007 (from now on shortly called PS) is a generator for the SopII-HtrII photosensor from Natronomonas Pharaonis coupled to E.Colis chemotaxis pathway via the Salmonella protein Tar. This part's effect on the system is to make E.Coli phototactic, so that it becomes aware of different light conditions. So for characterization of this part we made examination of motility and motility patterns our first priority and plasmid stability and growth of the cells our second.
There is a wide range of motility assays for chemotaxis in bacteria, which meant that we had a broad spectrum of experiments to choose from, which just had to be tweaked for making them suited for the analysis of phototaxis. The two experiments we chose for analysing the effect of this part (PS), were growth of the bacterial cultures in semi-solid agar and computer analysis of swimming motility through video microscopy:
1. Growth of bacterial culture on semi-solid agar plates (Experiment 1):
Since exposure to blue light should decrease the phototaxic bacterias tumbling frequency, the expected result was that the colony which was placed between the light and dark half of the plate would spread out in the darkness and would not move further when it reached the light. This is counter-intuitive, since decreased tumbling should lead to a longer distance traveled. What happens at the microscopic scale in semisolid agar is that the agar creates a matrix like structure where there are channels through the agar, which the bacteria can swim through. The decrease in tumbling frequency of the bacteria will make it harder for them to find the channels in the agar to swim through, which leads to them being trapped where they were placed. The result is that a colony which shows an increased run time, will look as if it it was non-motile on these plates. Our results showed exactly this; the bacterial culture had spread out to on the dark half of the plate and did not get nearly as far on the half exposed to light. This experiment was done with a normal wildtype MG1655 and a non-motile strain of E.coli, DH5alpha, as controls. As expected these cells did not show anything like the behavior described above, which indicates that the effect stems from the modification to our photosensor bacteria. These results were useable, but not fully conclusive, since there were some non-optimal conditions present in this experiment. We used ambient light instead of pure blue light, and the exposure to light for the multiple samples was not exactly even. Therefore we had to improve our experiment setup and see if we could reproduce these results with a more reliable setup.
2. Growth of bacterial culture on semi-solid agar plates (Experiment 2):
Another deviation from the protocol PS1.1 is that the culture was inoculated at the center of the plate, instead one colony each was inoculated in the center of the light exposed and dark half respectively. This would give us more information on how the bacteria would behave when directly exposed to either the dark or light surroundings, instead of the gradient that was present in the first experiment.
From the results of the first experiment we expected the culture containing K343007 to spread out in the dark and not to spread when exposed to light. The wildtype bacteria should spread out evenly no matter if exposed to light or not and the non-motile strain (DH5alpha) should not move regardless of the light conditions. Our expectations from the first experiment were fulfilled, as the bacteria behaved exactly as expected. This made it possible to conclude that the photosensor has an effect on the bacteria's tumbling frequency, but if it does in fact reduce the tumbling is not possible to say, since both an increased and reduced tumbling frequency will look alike.
Left to right: MG1655, Photosensor bacteria, DH5alpha (left half was in the dark, right half was exposed to light)
After this we could conclude that the part had an impact on the bacterial motility, but we had to find out which. This lead us to our next experiment, which was intended for determining what happened to the tumbling frequency:
3. Videomicroscopy and computer analysis of bacterial motility:
To get an exact idea of what is happening to the bacteria when expressing the photosensor we decided on doing video microscopy of motile bacteria. The bacterial cultures were prepared acording to protocol PS1.1. Wildtype MG1655 and DH5alpha was used as controls.
The actual microscopy was done on a (insert name) microscope with an optical magnification of 1000x.
To be able to observe an effect in the modified bacteria we had to expose the cultures to light, which was done through the microscope's integrated light source, which could switch between blue, green and ambient light. To ensure that the blue light was at the correct wavelength the same optical filter that was used for the plate experiments was inserted between the lightsource and the microscopy slide. Since microscopy is impossible without light, we had to use red light as a replacement for darkness, the source of the red light was ambient light from the microscope's light source filtered through a red optical filter (about 580nm). This should not have any effect on the phototactic bacteria according to Jung et. al (insert reference!). The room where the experiment was carried out was kept as dark as possible, as to eliminate the chance of the phototactic bacteria being unwantedly exposed to light. The actual experiment was carried out in the following manner:
1. Expose bacteria to red light for 1 minute, while recording video.
2. Move to another spot on the slide, repeat 1. (repeated until 5 videos were recorded)
3. Expose bacteria to blue light for 1 minute, while recording video.
4. Wait for 30 seconds, for the bacteria to reset themselves.
5. Move the focus to another spot on the slide, repeat 3. (x5)
This was done with all three strains of bacteria.
An example of the results:
When played at real time speed these videos seem slow and not showing any tendency, though when played at 4 times the normal speed, the photosensor will seem to show slightly increased motility when exposed to blue light. Even though the bacteria displayed disappointing motility, we went on and did computer analysis on the videos by the help of the open source software [http://db.cse.ohio-state.edu/CellTrack/ "CellTrack"]. The paths that were mapped showed a longer traveling distance for the bacteria expressing the photosensor compared to the rest of the samples when exposed to blue light. We picked 10 bacteria from each sample, which amounts to 60 bacteria that were tracked. This is a rather small amount, but because of the time-intensive procedure a more thorough data analysis was not possible.
Samples of the tracked paths (from left to right): Photosensor exposed to blue light, photosensor exposed to red light, wildtype exposed to blue light.
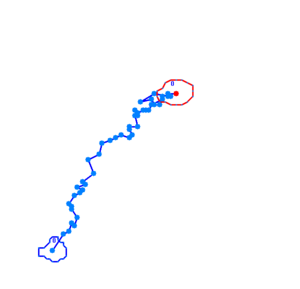
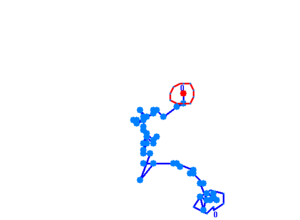
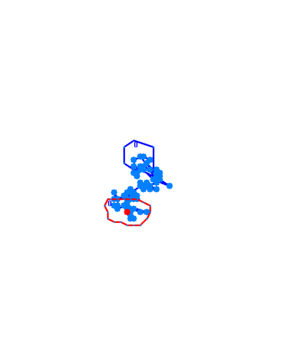
This might indicate a decreased tumbling frequency, but the low motility of the bacteria, the inadequacy of the cell track software and the small amount of data analysed make results of this experiment unreliable and a new protocol had to be devised.
4. Computerized analysis of the bacterial motility with the THOR prototype by [http://unisensor.dk/ Unisensor A/S] and the Unify software:
For this experiment we changed our protocol for cultivating swimming bacteria in order to optimize their mobility PS1.2
The machine and software used for the analysis are prototypes and still under heavy development. Because of trade secrets it is impossible for us to explain how the machine works or give a detailed explanation of it's mechanism until the THOR has reached production status. Simplistically said it is a very advanced video microscope, with which it is possible to analyse liquids and the particles inside in both 2D and 3D, while also tracking them over time.
2.5 ul of the bacterial dilution were placed on the center of the microscopy slide and covered by a coverslide. Afterwards the sample was sealed with mineral oil as to prevent a flow in the liquid. The data collection was done exactly as described in the previously experiment, with the exeption that instead of optical filters we used diodes that emitted light at the wanted wavelengths (500nm and 600nm respectively). Moreover we also recorded the bacteria's behaviour when exposed to a blue light gradient and varying intensities of light measured in milliampere. All the measurements were carried out on the modified phototactic bacteria and the wildtype MG1655.
The results look like these:
The bacteria that are not moving in the video are assumed to be sticking to the glass or coverslip.
5. Stability assay:

| Number of generations | 0 | 20 | 40 | 60 | 80 | 100 |
| Frequency of bacteria carrying plasmid | 100 | 61.33 | 94.40 | 97.16 | 120.38 | 121.55 |
| Standard deviation | 0 | 2.95 | 2.23 | 10.64 | 22.87 | 4.88 |
6. Growth measurement:
The purpose of this assay is to see if our transformants deviate from the wild type in growth rate. In the growth measurement assay we have measured OD at 550 nm every hour for 12 hours and at hour 24. In the experimental setup we used we see no lag phase in any of the measurments.
The graph below shows the growth of our wild type E. coli strain MG1655, the MG1655 transformed with K343007 in pSB3T5 and in pSB1C3.

From our data we see no significant difference between the transformed bacteria and the wild type.
K343005
1.UV-Vis determination of beta-carotene and retinal production:
In this experiment cells was prepare and harvested according to protocol [1]. This experiment was preformed with six different strains of E. coli:
Wild type Top10
Wild type MG1655
Top10 containing PSB1A2 with constitutively active K274210
MG1655 containing PSB1A2 with constitutively active K274210
TOP10 containing PSB1A2 with K274210 and PSB1C3 with K343005, both under a constitutively active promotor
MG1655 containing PSB1A2 with K274210 and PSB1C3 with K343005, both under a constitutively active promotor
The measurements were performed on cells after 20 hours of growth seeing as the first experiment showed no or little product in the exponential phase. The resulting graphs are presented below:
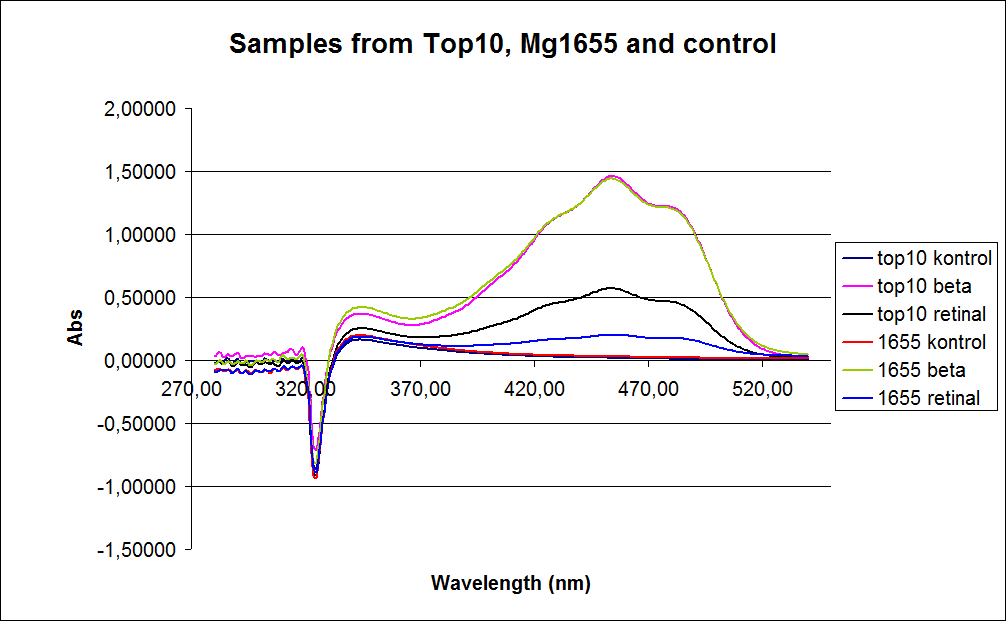
In the spectrum, a sudden drop at 320 nm occurs. This cannot be explained .
The graph also shows that some cell material interferes with the UV-vis measurement where the retinal has the strongest absorption. For this reason, some organic separation may be needed before the measurement is performed.
In order to assess the results, standard dilutions of beta-carotene and retinal were made and measured. The concentrations were 1mM, 100 µM, 50 µM, 25 µM, 10 µM, 5 µM, 1 µM, 100 nM and 10 nM. The standard dilutions were measured on the spectrophotometer. The resulting graphs are presented below:
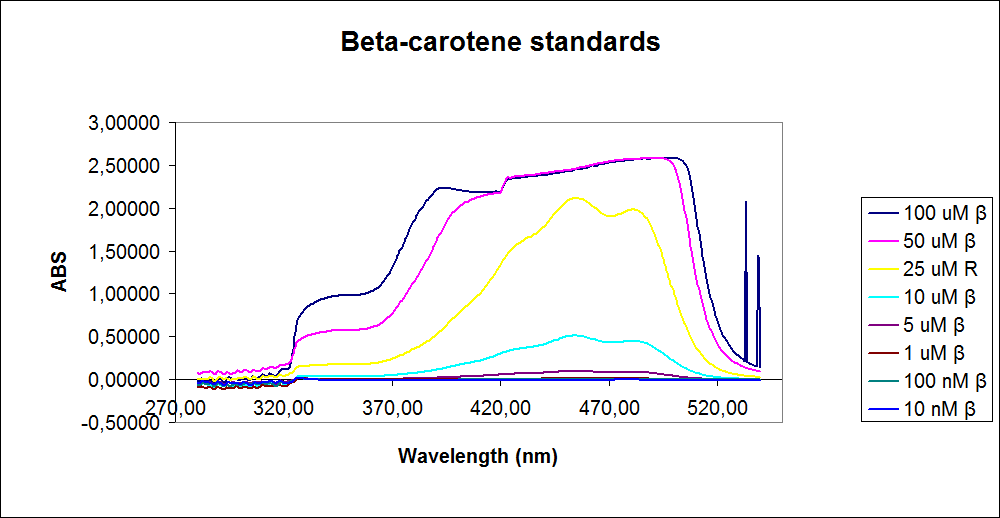
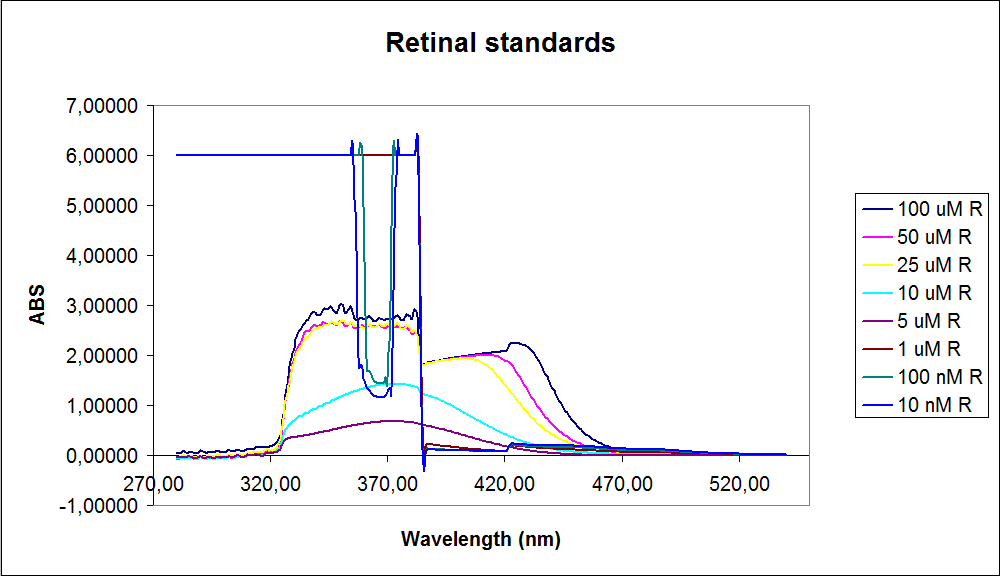
The spectrum for the beta-carotene looks normal and reliable, the standards for retinal, however, don’t look reliable. The spectrum for the three lowest concentrations appear to be giving a wrong picture from the measurement.
The measurement jumps rapidly from nearly nothing to the maximum absorbance. This might be because the concentration of retinal is too low to be measured as a spectrum, but the concentration of the individual derivates of retinal might be high enough to be measured.
It might also be that the concentrations are too low and something else is influencing the measurements.
Because of the error in the UV-vis spectrum concerning retinal detection in both the standards and samples, the next characterization experiments are preformed on a HPLC.
K343006
1. HPLC determination of beta-carotene and retinal production:
2. Stability assay:
3. Growth measurement:
The purpose of this assay is to see if our transformants deviate from the wild type in growth rate. In the growth measurement assay we have measured OD at 550 nm every hour for 12 hours and at hour 24. In the experimental setup we used we see no lag phase in any of the measurments.
The graph below shows the growth of our wild type E. coli strain MG1655, the MG1655 transformed with K343006 in pSB3T5 and in pSB1C3.

From our data we see no significant difference between the transformed bacteria and the wild type.
Helping another iGEM Team with characterization
2010 Bielefeld brick K389016
We recieved a miniprep with [http://partsregistry.org/Part:BBa_K389016 K389016] in pSB1C3 together with a list of experiments and characterization assays the Bielefeld team would like us to carrie out.
We transformed the plasmid into E. coli strain MG1655 and ran a colony PCR of four different colonies the following day. The picture below shows that cells in all four colonies contained K389016 in pSB1C3.
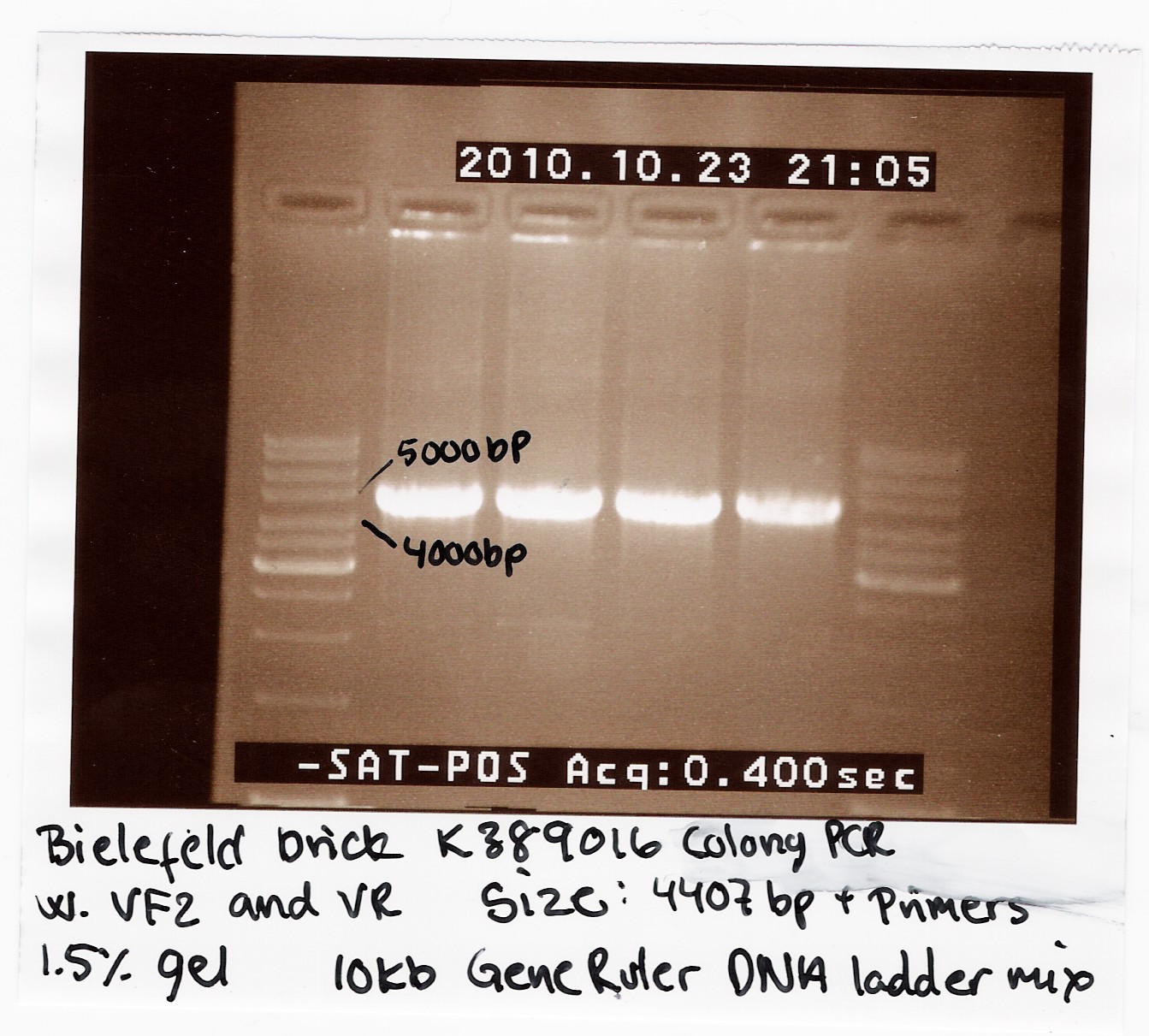
Characterization
1. Growth assay:
Characterizing an exicting biobrick
2009 Cambridge brick K274210
The K274210 brick was cultivated from the 2010 kit plate 3 well N6 in the PSB1A2 plasmid and transformed into E. coli strains TOP10 and MG1655.
Characterization
1. UV-vis determination of beta-carotene production:
In this experiment cells were prepared and harvested according to protocol [2]. This experiment was performed with four different strains of E. coli:
Wildtype Top10
Wildtype MG1655
Top10 containing PSB1A2 with constitutively active K274210
MG1655 containing PSB1A2 with constitutively active K274210
The measurements were performed on cells both in the exponential and stationary phase. The resulting graphs are presented below:
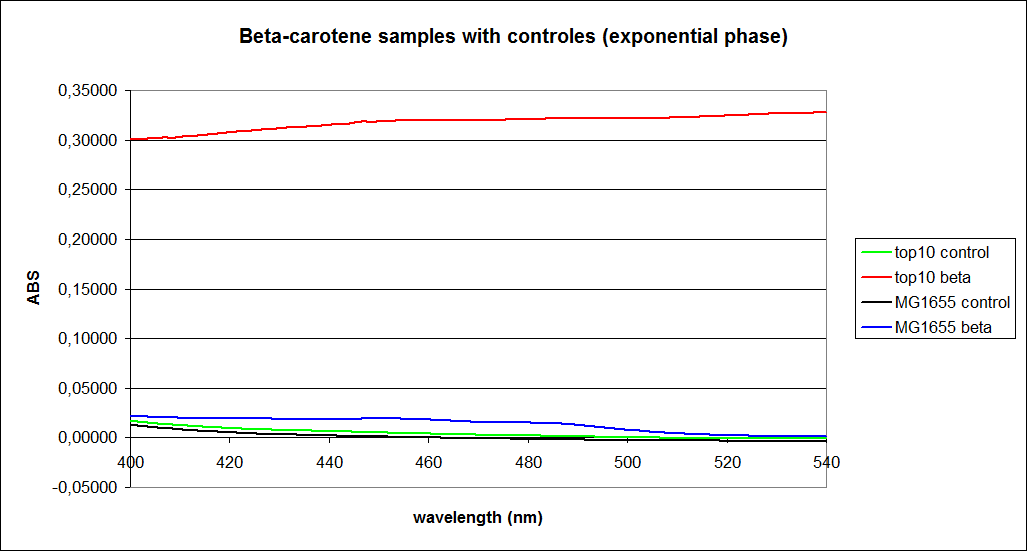
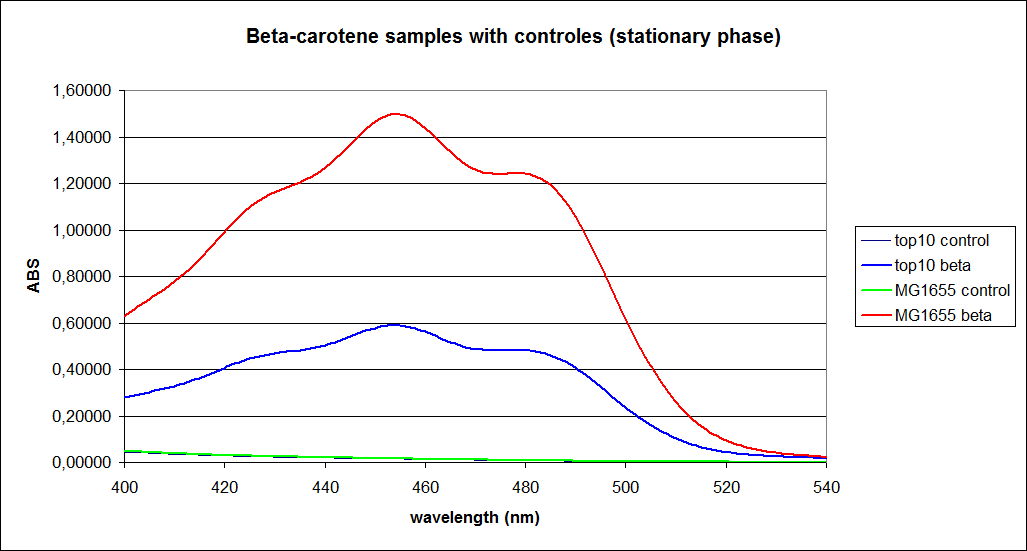
In order to assess the results, a series of standard dilutions of beta-carotene were made. The concentrations were 1mM, 100 µM, 50 µM, 25 µM, 10 µM, 5 µM, 1 µM, 100 nM and 10 nM. The standard dilutions were also measured on the spectrophotometer. The resulting graphs are presented low:
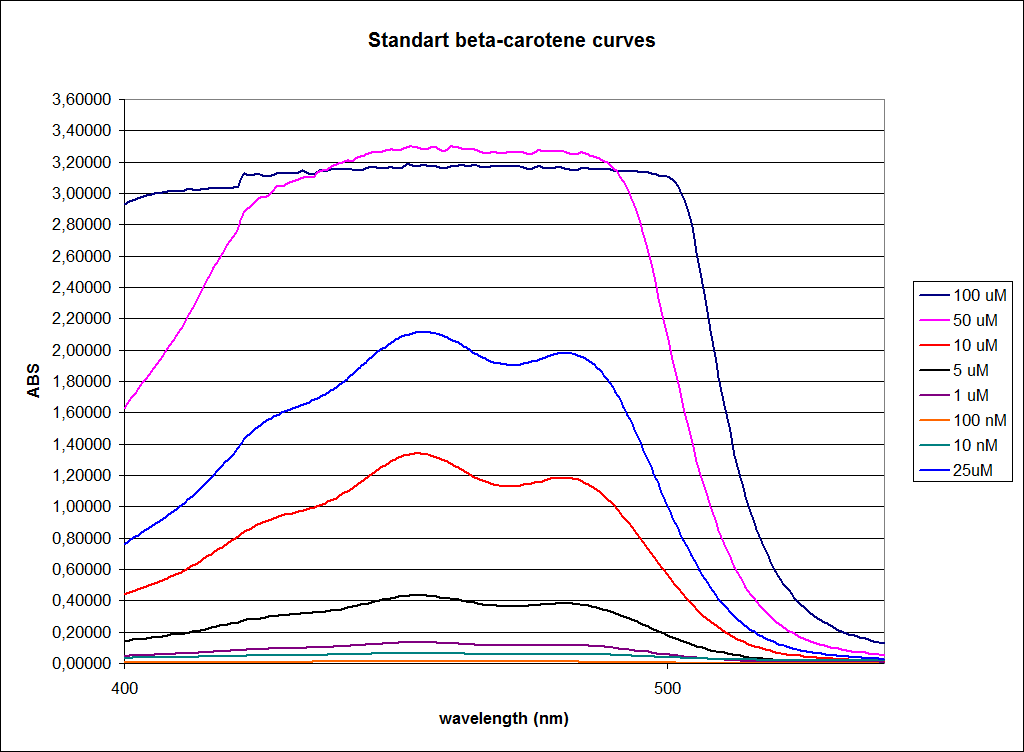
When combining the graphs of the samples and the standard dilutions, it is clear that the spectres are uniform. This similarity of the spectres indicates that the cells actually do produce beta-carotene. The graphs also give a qualitative indication of the amount of beta-carotene produced by the MG1655 and the Top10 E. coli cells. The resulting graphs are presented below:
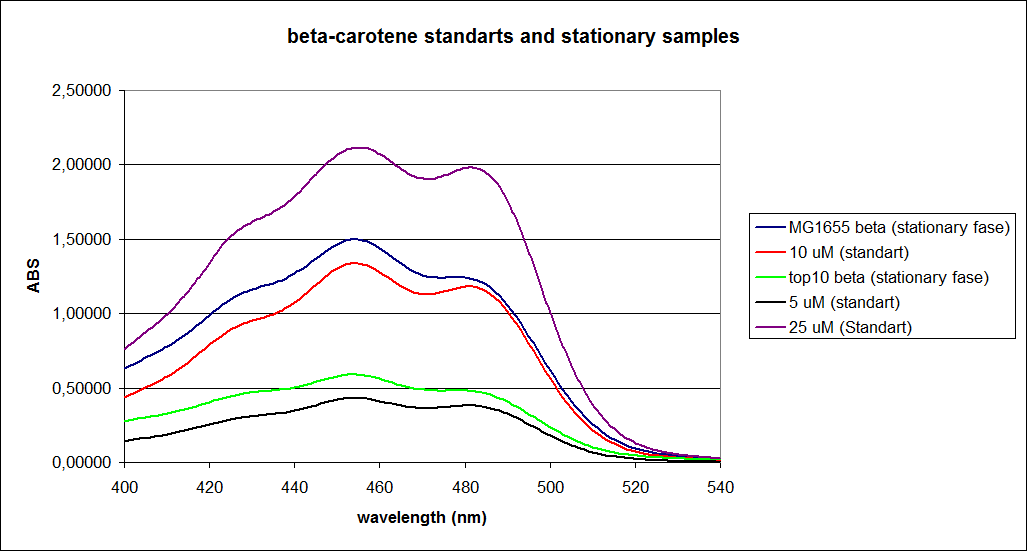
 "
"