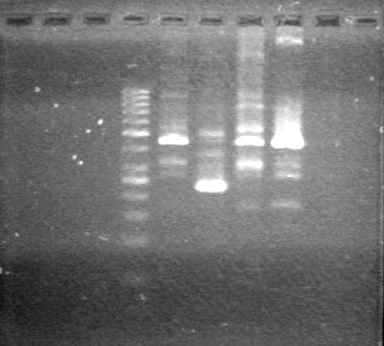
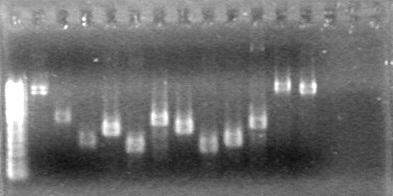
| Lane | Content | Quantity of DNA (ng/µL) |
| 1 | 1kB Ladder | 12.5 |
| 3 | pLacI restriction digest | 5.21 |
| 5 | sRBS restriction digest | 3.72 |
| 7 | xylErestriction digest | 3.36 |
| 9 | Lumazine restriction digest | 2.63 |
| 11 | dT restriction digest | 2.98 |
July 19, 2010 Evening
(K.G)
Objective: Ligate together rbs-xylE and dT, lumazine and dT, also pLacI and sRBS.
Method: All ligation mixes had:
- 10X T4 Ligation Buffer
- Milli-Q H2O to fill to 20µL
- T4 DNA Ligase
- 20ng plasmid DNA
Ligations were left over-night at room temperature.
(J.V.)
Objective:Determine the results of the transformations done by K.G. on July 15/2010.
Method: Inoculate 5mL LB media w/Amp and colony. Incubated over night at 37oC.
Results: Lumazine Synthase with dT ligated onto it grew.
July 20, 2010
(AV, HB)
Objective:Miniprep lumazine-dt and 4 N-terminus tags and analyze.
Method:
- Use boiling lysis miniprep to isolate plasmid DNA.
- PCR amplify BioBrick part to determine if the correct DNA was isolated.
- Ran a 1% Agarose gel to visualize PCR products.
Results:
Agarose gel did not show any bands.
July 20, 2010 Evening
(AV, HB)
Objective:Transform ligation done on July 19, 2010 in to competent DH5α cells.
- xylE-dT
- lumazine synthase-dT
- pLacI-sRBS
- EYFP and ECFP
Method:
Results:
- xylE-dT no colonies
- pLacI-sRBS
- EYFP
- lumazine-dT
- ECFP
July 21, 2010
(JV)
Objective: Isolate plasmid DNA from pTet, TetR, pET-28(a).
Method:
(JV)
Objective: To induce over-expression of pLacI-RBS-Mms6-dt in BL21(DE3) cells.
Method: Used Overexpression.
Ran SDS gel for 78 minutes at 200V
July 21, 2010 Evening
(TF, AS)
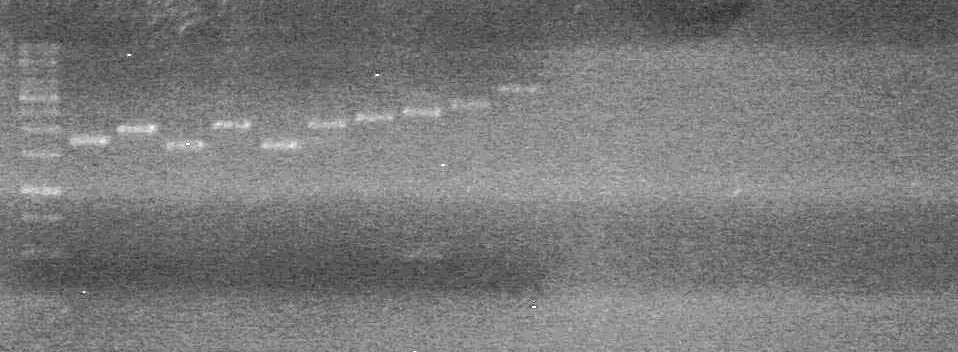
Objective: Colony PCR to test for insertions of BioBrick construction, and for quality control of parts received from the Registry. Also to prepare DNA to be sent away for sequencing.
Method:
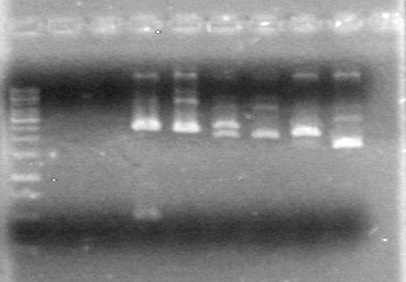
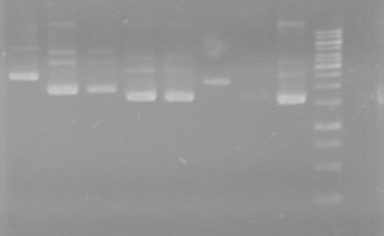
-Ran PCR's
-Ran 2% Agarose gel for:.
- K249001
- K249004
- K249005
- K249006
- K249008
- K249014
- K249017
- mms6-dt
- lumazine-dt
- ECFP
- EYFP
- N-term tag
- xylE-dt
- SRBS-pLacI
- dt
- pTet maxiprep
- pET-28(a) maxiprep
- TetR maxiprep
Gel ran at 100V for 60minutes.
Results: The PCR's did not work. However, the maxipreps show DNA
July 23, 2010
(JV)
Objective: Insert xylE, Mms6, and lumazine synthase into pET-28(a).
Method: A restriction digest was performed on xylE and Mms6 and ran for 90 minutes at 37 C
Results:Did not see any cut out biobricks.
July 27, 2010
(in lab: JV, AV, HB)
Objective: To miniprep the overnight cultures of the parts ordered from the Parts Registry and to test the efficiency of the Qiagen Miniprep Kit.
Method:
The following parts were miniprep'd using Boiling Lysis Plasmid Preparation:
- dT (control)
- E0020 (ECFP)
- E0030 (EYFP)
- K249001
- K249004
- K249005
- K249006
- K249008
- K249014
- K249017
The following parts were miniprep'd using Qiagen Miniprep Kit:
Results: Qiagen Miniprep Kit produced a comparable quantity of DNA to Boiling Lysis Plasmid Preparation. Since the Qiagen Miniprep Kit is faster and easier, all minipreps will be done using the Qiagen Miniprep Kit.
July 28, 2010
(in lab: JV)
Objective: To create a large quantity of pSB1C3 plasmid.
Method: PCR amplified pSB1C3 received from iGEM headquarters.
July 29, 2010
(in lab: HB, AV)
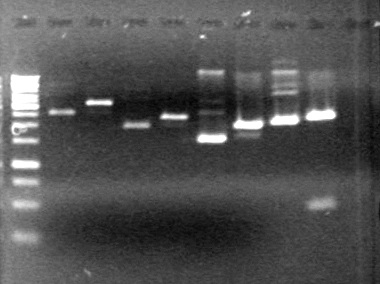
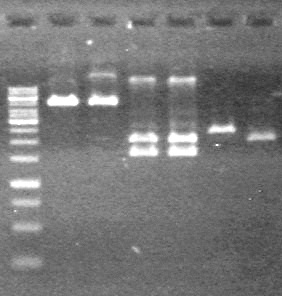
Objective: To PCR amplify the minipreps on July 27, 2010.
Method: PCR amplified the 11 minipreps and dT control.
Results: PCR amplification was successful and 0.2 µL of polymerase will be used per PCR reaction in the future.
Objective: To maxiprep EYFP (E0030), ECFP (E0020), and Lumazine Synthase (K249002).
Method: Used Maxiprep
| Cell Pellet | Weight (g) |
| ECFP | 2.32 |
| EYFP | 2.00 |
| Lumazine Synthase | 2.66 |
July 29, 2010 Evening
(in lab: KG)
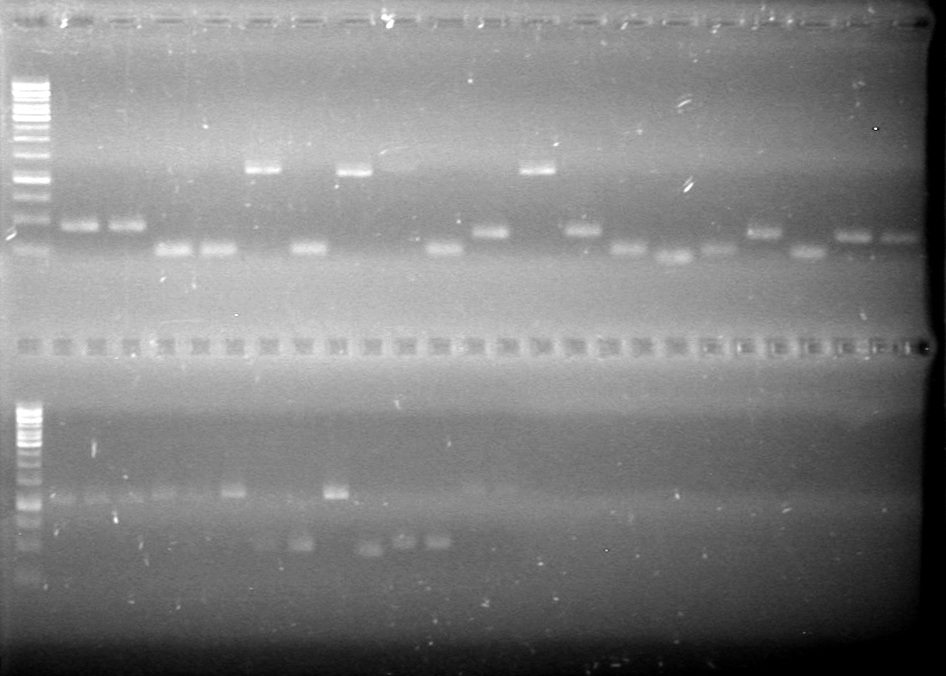
Objective: Run PCR products from the morning on a 2% agarose gel.
Method: Ran 2% agarose gel of the 11 minipreps and dT control and stained in EtBr for 17 minutes.
Results:PCR amplification of last year's parts not consistent with sizes listed on registry.
 "
"