Team:Monash Australia/Modelling
From 2010.igem.org
(→Model 1) |
(→Model 1) |
||
| Line 21: | Line 21: | ||
This specific rate is defined by the Michaelis-Menton Equation | This specific rate is defined by the Michaelis-Menton Equation | ||
| - | + | <math>kcat*[substrate]*[enzyme]</math> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
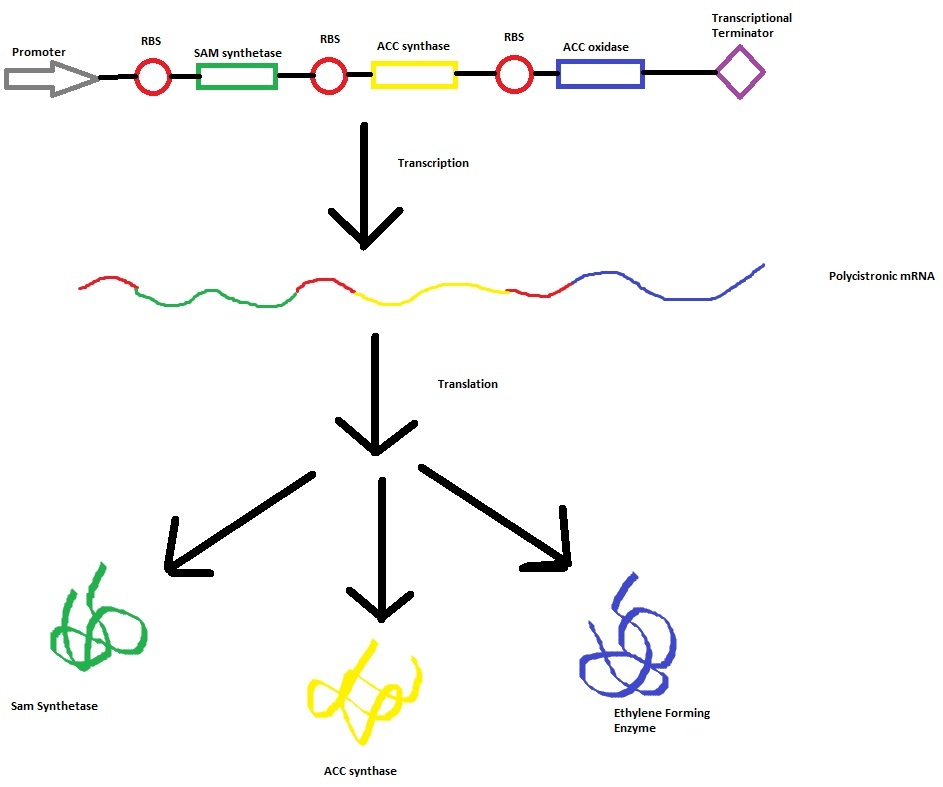
== Construct --> mRNA --> enzymes (SAMsynth, ACCS, EFE) == | == Construct --> mRNA --> enzymes (SAMsynth, ACCS, EFE) == | ||
Revision as of 12:20, 26 October 2010

|
|
|||
Contents |
Introduction
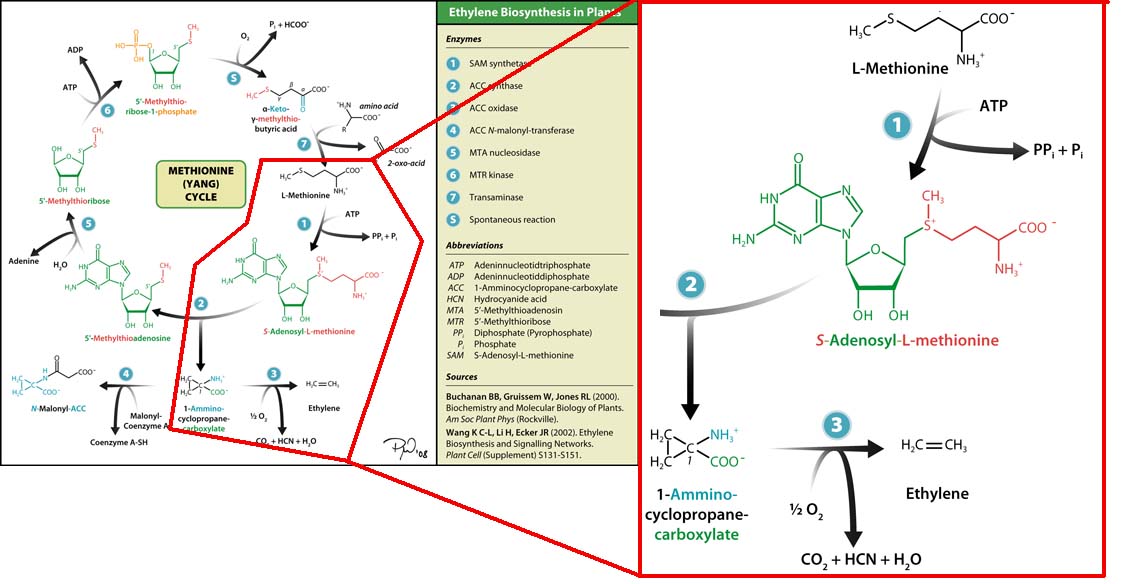
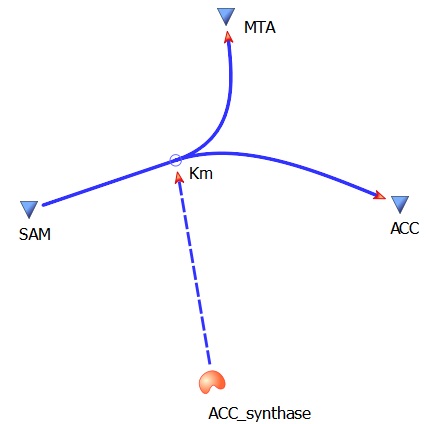
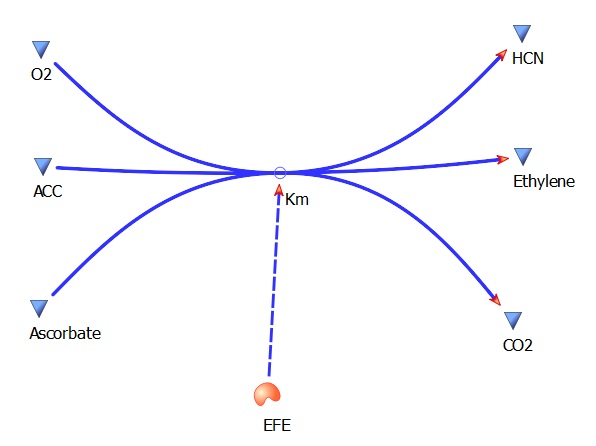
The three key enzymes we require are highlighted in the image, SAM synthase, ACC synthase and ACC oxidase. SAM synthase converts methionine into S-Adenosyl-L-Methionine (SAM), using ATP for an adensoyl group. The second step involves ACC synthase, which cleaves the amino butyrate from SAM, releasing 1-aminocyclopropane-1-carboxylic acid (ACC). Released ACC is then processed by ACC Oxidase which converts ACC to ethylene by cleaving the carboxylic acid off as carbon dioxide and its neighboring carbon with the amino group as cyanide gas. By using such a system to produce ethyene gas we can potentially reduce costs involved with current production methods by reducing temperature requirements by 30 fold.
Kinetic modelling of the ethylene generator
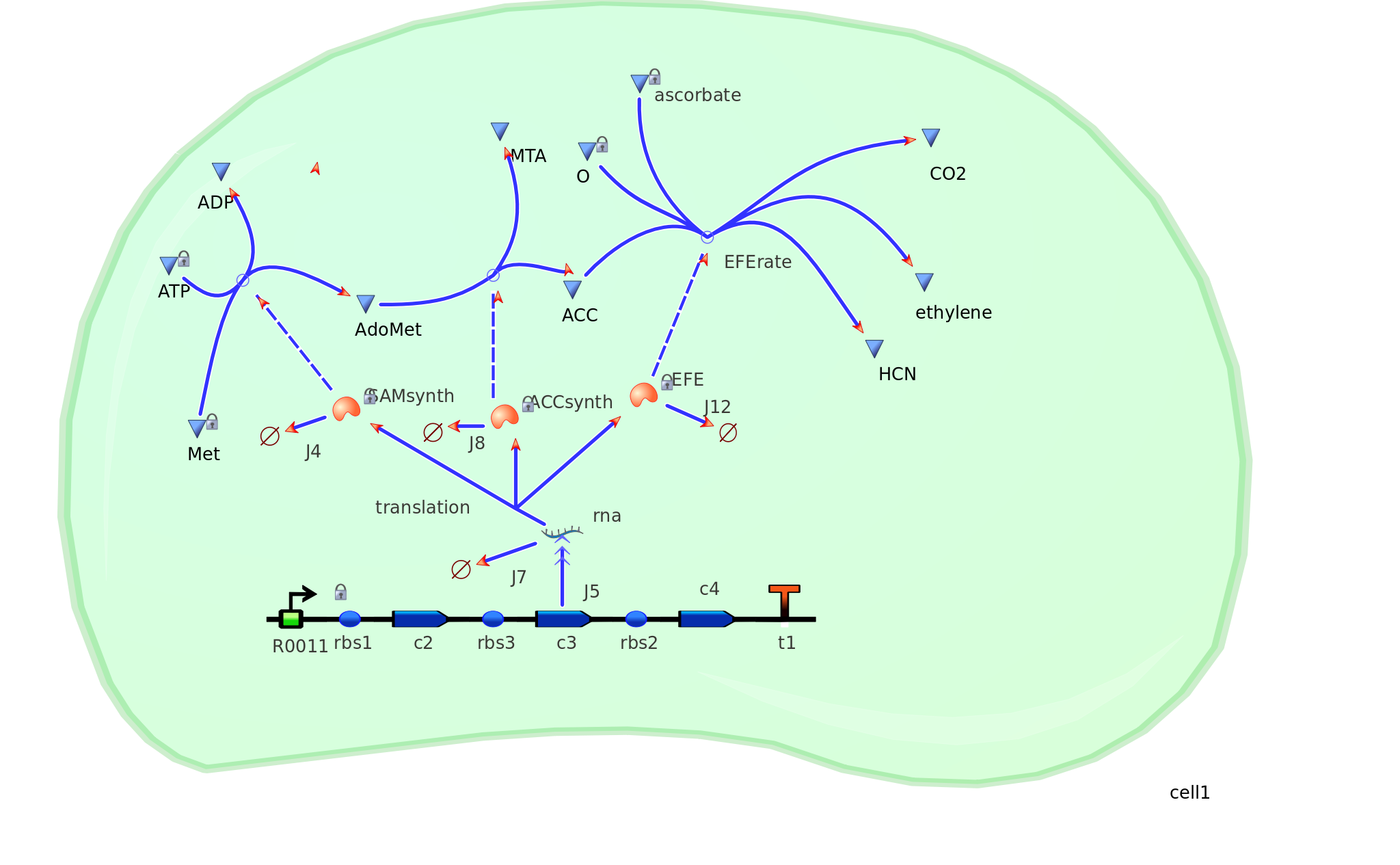
The program tinkercell was used to model the synthesis of ethylene in our e.coli cell. Where, in addition to the qualitative representation (figure 1) the program allowed, simulation of the model could be exercised inorder to measure the flux of metabolites – with respect to time - in the system given the input of values pertaining to substrate/enzyme concentration, and reaction rates were inputted. In addition to experimental data, with a model, theoretical data can be obtained using the model to extrapolate quantitative data corresponding to mRNA, protein and ethylene outputs under set parameters and assumptions in silico. As a result, using the given theoretical model outputs as a benchmark, manipulations to the parameters and assumptions set can be made in vitro/in vivo in order to achieve higher outputs in future studies of the 'ethylene generator'.
Model 1
This model was designed to be the starting point of the kinetic modelling. Therefore it was decided to begin with a realitivly simple construct and implement it to obtain an ethylene output. This design comprised only of three enzymes. SAM, ACC synthase and ACC oxidase or EFE (ethylene forming enzyme). These enzymes were presnt at fixed concentrations and in the 1:1:1 ratio. Concentrations included 10, 100, 1000 and 3000 uM. This ensured that the model was basic and ultimately obtained a resonable yet simple ethylene output. Therefore by having this straightforward design with constant concentrations , ethylene output is only based on the rate of the specific enzymes.
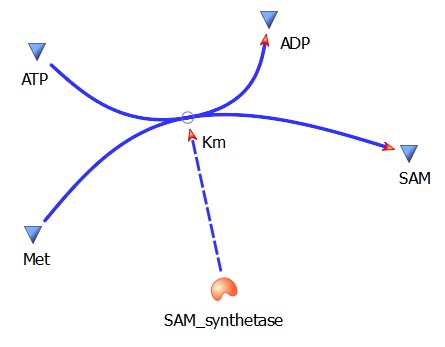
This specific rate is defined by the Michaelis-Menton Equation
<math>kcat*[substrate]*[enzyme]</math>
Construct --> mRNA --> enzymes (SAMsynth, ACCS, EFE)
Met --> SAM (AdoMet)
SAM --> ACC
ACC --> Ethylene
More to come soon!
 "
"