Team:Washington/Tools Created/New Vectors
From 2010.igem.org
(→Testing the Vectors) |
(→Protein Expression) |
||
| Line 100: | Line 100: | ||
[[Image:Washington2010_vector_assay_data.png|thumb|left|650px|]] | [[Image:Washington2010_vector_assay_data.png|thumb|left|650px|]] | ||
The vector cassettes was placed in 4 different plasmid backbones from the registry [http://partsregistry.org/Part:pSB1C3 pSB1C3], [http://partsregistry.org/Part:pSB1A3 pSB1A3], [http://partsregistry.org/Part:pSB3K3, pSB3K3], [http://partsregistry.org/Part:pSB4A5 pSB4A5] and [http://partsregistry.org/Part:BBa_E0040 GFP] was placed in the protein expression area of the vector. The expression cassette was grown according to [https://2010.igem.org.wiki/Team:Washington/protocol/vector_assay vector assay]. Data was corrected for background florescence. [[Image:Washington_f1_origin_gel.png|thumb|right|300px|]] | The vector cassettes was placed in 4 different plasmid backbones from the registry [http://partsregistry.org/Part:pSB1C3 pSB1C3], [http://partsregistry.org/Part:pSB1A3 pSB1A3], [http://partsregistry.org/Part:pSB3K3, pSB3K3], [http://partsregistry.org/Part:pSB4A5 pSB4A5] and [http://partsregistry.org/Part:BBa_E0040 GFP] was placed in the protein expression area of the vector. The expression cassette was grown according to [https://2010.igem.org.wiki/Team:Washington/protocol/vector_assay vector assay]. Data was corrected for background florescence. [[Image:Washington_f1_origin_gel.png|thumb|right|300px|]] | ||
| + | <br /> | ||
| + | <br /> | ||
<br /> | <br /> | ||
<br /> | <br /> | ||
Revision as of 02:46, 23 October 2010
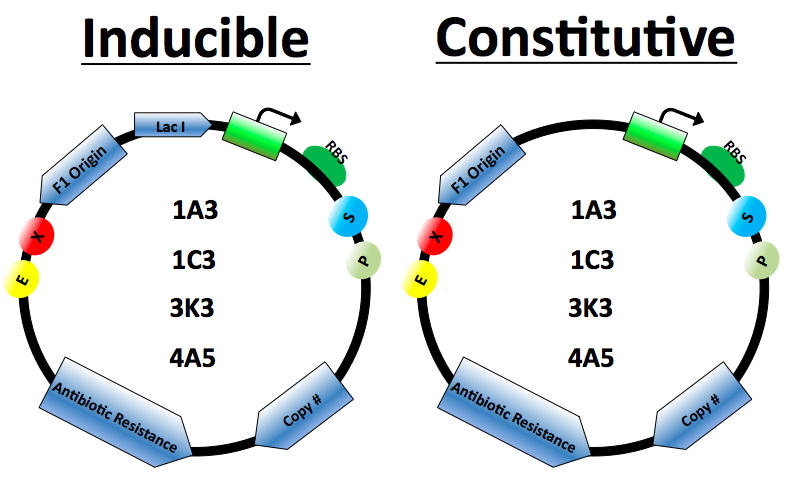
Protein Expression Vectors
Vector Design
The protein expression cassette is designed to go into any biobrick plasmid backbone. It was placed in 4 different plasmid backbone from the registry [http://partsregistry.org/Part:pSB1C3 pSB1C3], [http://partsregistry.org/Part:pSB1A3 pSB1A3], [http://partsregistry.org/Part:pSB3K3, pSB3K3],and [http://partsregistry.org/Part:pSB4A5 pSB4A5] by our lab.
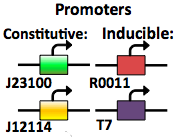
The expression vector promoters are available in constitutive and inducible variety. The [http://partsregistry.org/Part:BBa_J23100 Constitutive promoters] are BBa J23100 and J23114. Inducible vectors include [http://partsregistry.org/Part:BBa_R0011 R0011] and [http://partsregistry.org/Part:BBa_K314112 T7], both of which are repressed by the Lac I protein.
Lac I gene inserted to ensure regulation of the Lactose inducible promoters
Antibiotics resistance based on biobrick plasmid backbone used.
The Elowitz standard RBS [http://partsregistry.org/Part:BBa_B0034 B0034] is used on all vectors.
Origin of replication for the M13 series of phages. When included in plasmids that are infected by M13 helper phage will generate SS DNA of the plasmid.
Plasmid copy number based on biobrick plasmid backbone used.
Restriction cut sites based on biobrick standards.
Building the Vectors
The expression cassettes were built using the [http://openwetware.org/wiki/BioBricks_construction_tutorial biobrick construction tutorials].
Testing the Vectors
Protein Expression
The vector cassettes was placed in 4 different plasmid backbones from the registry [http://partsregistry.org/Part:pSB1C3 pSB1C3], [http://partsregistry.org/Part:pSB1A3 pSB1A3], [http://partsregistry.org/Part:pSB3K3, pSB3K3], [http://partsregistry.org/Part:pSB4A5 pSB4A5] and [http://partsregistry.org/Part:BBa_E0040 GFP] was placed in the protein expression area of the vector. The expression cassette was grown according to vector assay. Data was corrected for background florescence.
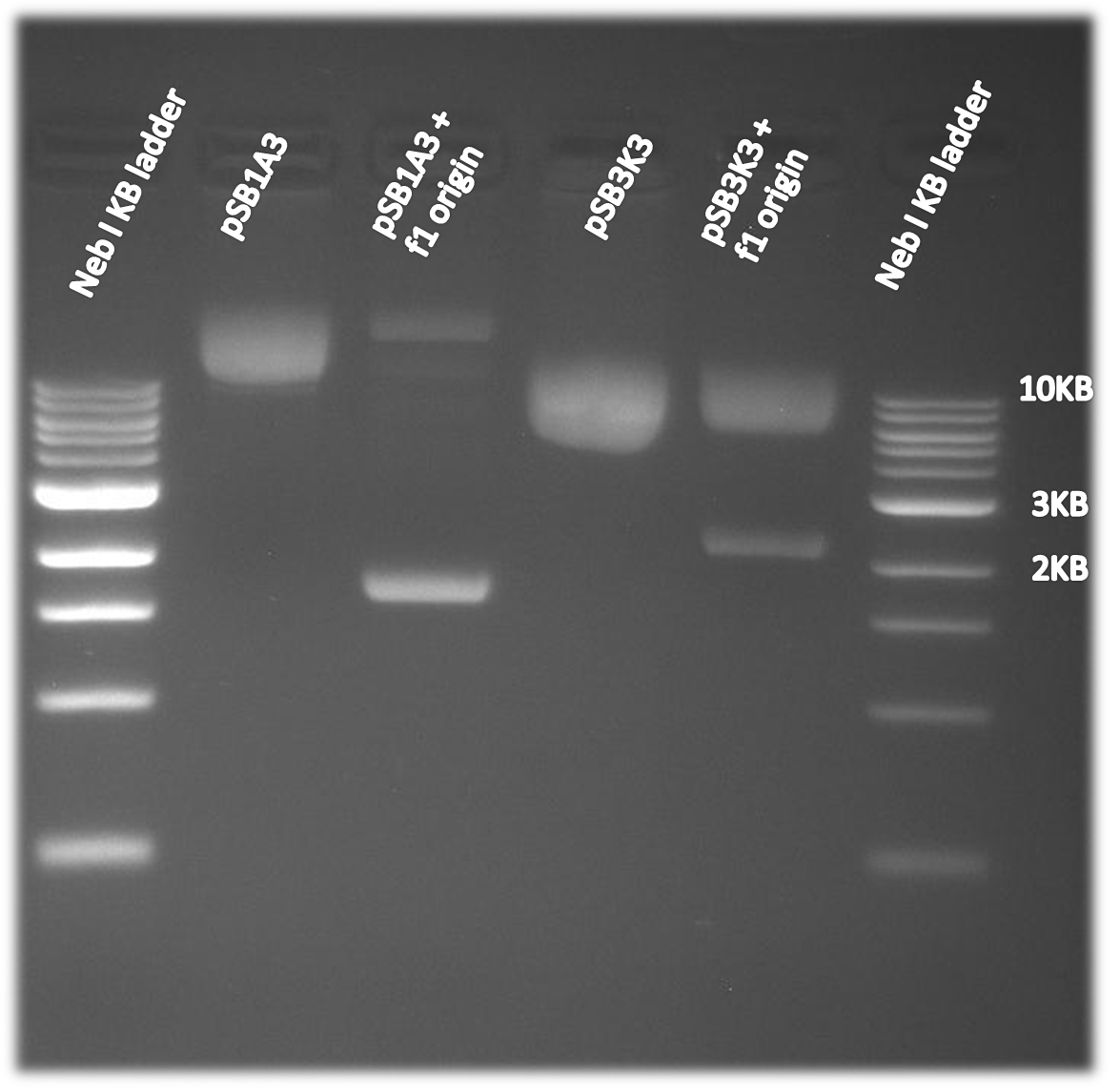
f1 origin
The f1 origin was tested by comparing single strand DNA harvest using part of the Kunkel's mutagenesis. CJ236 cells were infected with M13K07 helper phage. The CJ236 cells varied in the present or absence of the f1 origin on the pSB1A3 or pSB3K3 plasmid. The SS DNA harvest was then run on a 50ml 1% agarose gel at 90V for 45minutes. As is clearly visible on the gel to the right single strand DNA of the plasmid is only made when the f1 origin is present.
 "
"