Team:Newcastle/24 August 2010
From 2010.igem.org
(→Results) |
(→Results) |
||
| Line 100: | Line 100: | ||
[[Image:24.08.10.png|500px]] | [[Image:24.08.10.png|500px]] | ||
| + | |||
| + | '''Figure 1''': Gel electrophoresis of the amplified PCR products and restriction digest | ||
| + | |||
| + | *'''Lane 1''': 1 Kb ladder | ||
| + | *'''Lane 2''': digested (linearised) pGFP-rrnB | ||
==Discussion and Conclusion== | ==Discussion and Conclusion== | ||
Revision as of 01:16, 26 October 2010

| |||||||||||||
| |||||||||||||
Contents |
Miniprep for pGFPrrnB with yneA
Aims
To produce more stocks of vector pGFPrrnB with insert yneA.
Materials and Protocol
Please refer to qiagen minipreps and nanodrop spectrophotometer for all 12 tubes of miniprep that we did.
Results
The results from the nanodrop:
| DNA | Concentration (ng/µl) |
|---|---|
| 1 | 286.1 |
| 2 | 304.0 |
| 3 | 316.3 |
| 4 | 421.6 |
| 5 | 518.7 |
| 6 | 460.1 |
| 7 | 370.1 |
| 8 | 377.3 |
| 9 | 346.0 |
| 10 | 347.4 |
| 11 | 202.8 |
| 12 | 307.4 |
Discussion
The results from the nanodrop showed that we have produced high concentration of vector pGFPrrnB with insert yneA. We will then proceed to digestion.
Single and Double Digestion of pGFPrrnB with yneA
Aims
To check if the insert yneA has been inserted into vector pGFPrrnB.
Materials and Protocol
We are doing two digests for pGFPrrnB and yneA:
- Single digest with HinDIII;
- Double digest with EcoR1 and Nhe1.
Please refer to restriction digests and gel electrophoresis.
Results
Gel electrophoresis results for digestion:
Discussion and Conclusion
Single digestion of pSB1C3
Aims
To linearise the vector pSB1C3. The linear vector will be used as a PCR template for Gibson cloning of the Subtilin immunity and rocF BioBricks.
Materials and Protocol
We are doing a single digest with HinDIII.
Please refer to restriction digests and gel electrophoresis.
Results
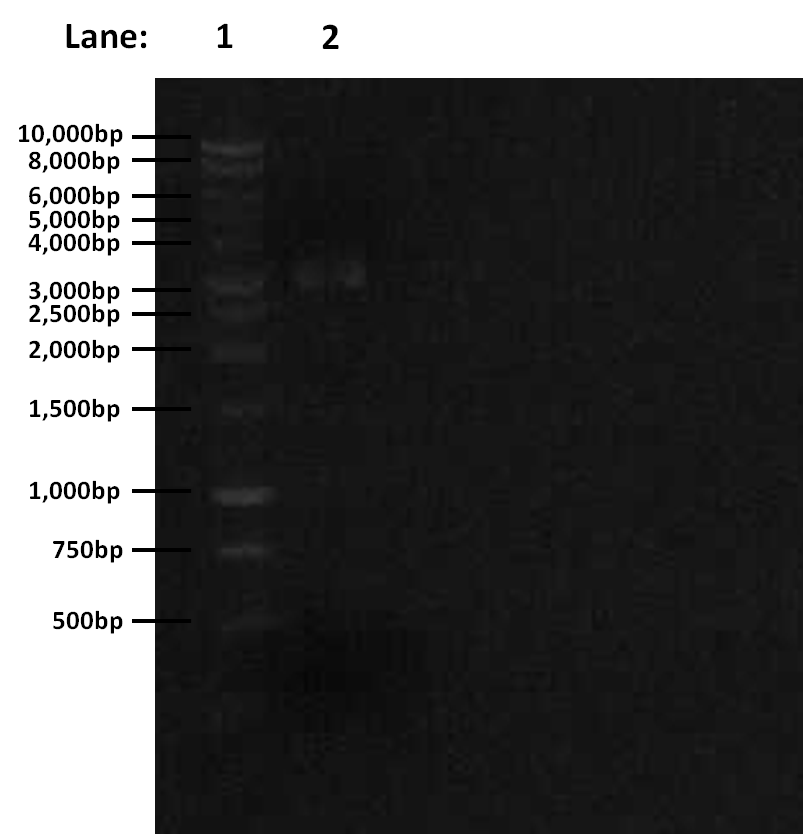
Gel electrophoresis results for digestion:
Figure 1: Gel electrophoresis of the amplified PCR products and restriction digest
- Lane 1: 1 Kb ladder
- Lane 2: digested (linearised) pGFP-rrnB
Discussion and Conclusion
The digestion was successful — the correct band was seen. We can proceed to gel extraction.
 
|
 "
"