Team:UNIPV-Pavia/Calendar/September/settimana5
From 2010.igem.org
m (→October, 2nd) |
(→October, 1st) |
||
| (47 intermediate revisions not shown) | |||
| Line 32: | Line 32: | ||
<br> | <br> | ||
| - | <html><p align="center"><font size="4"><b>SEPTEMBER: WEEK | + | <html><p align="center"><font size="4"><b>SEPTEMBER: WEEK 5</b></font></p></html><hr><br> |
<html><a name="indice"/></html> | <html><a name="indice"/></html> | ||
==September, 27th== | ==September, 27th== | ||
| + | <font size=4>[[Team:UNIPV-Pavia/Material Methods/Measurements/Tecan/test27settembre|Tecan Test]]</font> | ||
| + | |||
| + | ---- | ||
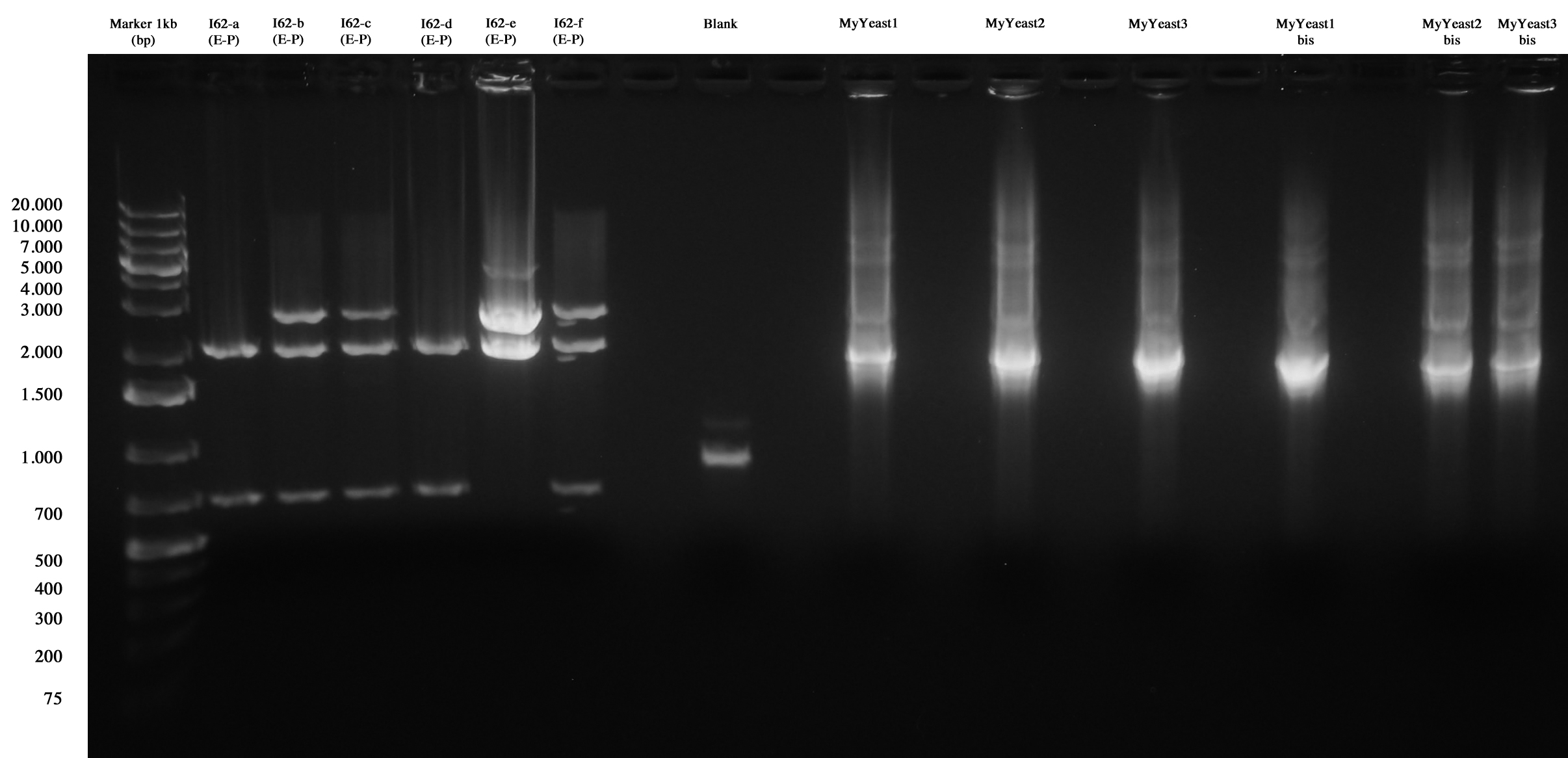
MiniPrep and E-P digest for I62-a, I62-b, I62-c, I62-d, I62-e, I62-f | MiniPrep and E-P digest for I62-a, I62-b, I62-c, I62-d, I62-e, I62-f | ||
| - | DNA | + | DNA PCR for MyYeast 1-2-3 |
Gel run | Gel run | ||
| - | [[Image:UNIPV10_27_09_2010_I62_screen.jpg|thumb|200px|center|I62 and MyYeast | + | [[Image:UNIPV10_27_09_2010_I62_screen.jpg|thumb|200px|center|I62 and MyYeast screening.]] |
| Line 48: | Line 51: | ||
| - | Protein electrophoresis for phasins | + | Protein electrophoresis for phasins. |
<div align="right"><small>[[#indice|^top]]</small></div> | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==September, 28th== | ==September, 28th== | ||
| - | <font size=4>[[Team:UNIPV-Pavia/Material Methods/Measurements/Tecan/test28settembre|Tecan Test]]</font> | + | <font size=4>[[Team:UNIPV-Pavia/Material Methods/Measurements/Tecan/test28settembre|Tecan Test]]</font> |
---- | ---- | ||
| Line 76: | Line 79: | ||
==September, 29th== | ==September, 29th== | ||
| - | |||
| - | |||
Colony PCR for 6 newly picked colonies. | Colony PCR for 6 newly picked colonies. | ||
| - | [[Image:UNIPV10_29_09_2010_I55_screen.jpg|thumb|200px|center|I55 colony PCR screening | + | [[Image:UNIPV10_29_09_2010_I55_screen.jpg|thumb|200px|center|I55 colony PCR screening.]] |
No positives nor this time. | No positives nor this time. | ||
---- | ---- | ||
| Line 86: | Line 87: | ||
Gel run for all digested parts | Gel run for all digested parts | ||
| - | [[Image:UNIPV10_29_09_2010_digestions.jpg|thumb|200px|center|Digestions | + | [[Image:UNIPV10_29_09_2010_digestions.jpg|thumb|200px|center|Digestions.]] |
| - | and | + | and cut/gel purification for: |
*4C5 (E-P): 19,3 ng/ul | *4C5 (E-P): 19,3 ng/ul | ||
*My Yeast 3 (E-S): 20,8 ng/ul | *My Yeast 3 (E-S): 20,8 ng/ul | ||
| Line 108: | Line 109: | ||
|} | |} | ||
| + | ---- | ||
| + | Inoculum of MC43, MG43 in 5ml LB with chloramphenicol concentration of 12.5 ug/ml. | ||
| Line 114: | Line 117: | ||
==September, 30th== | ==September, 30th== | ||
| - | <font size=4>[[Team:UNIPV-Pavia/Material Methods/Measurements/Tecan/ | + | <font size=4>[[Team:UNIPV-Pavia/Material Methods/Measurements/Tecan/test30settembre|Tecan Test]]</font> |
---- | ---- | ||
| Line 131: | Line 134: | ||
*I20: (E-X) | *I20: (E-X) | ||
| - | Gel run/cut/extraction | + | Gel run/cut/extraction/quantification |
[[Image:UNIPV10_30_09_2010_I20_E_X.jpg|thumb|70px|center|I20 E-X digest.]] | [[Image:UNIPV10_30_09_2010_I20_E_X.jpg|thumb|70px|center|I20 E-X digest.]] | ||
| - | I20 E-X: ng/ul | + | I20 E-X: 13,4 ng/ul |
| + | |||
We repeated I55 ligation using newly digested vector and already digested I38 (E-S) | We repeated I55 ligation using newly digested vector and already digested I38 (E-S) | ||
| Line 150: | Line 154: | ||
---- | ---- | ||
| - | Inoculum of E. coli TOP10 in 1,5 ml LB, grown ON at | + | Inoculum of E. coli TOP10 in 1,5 ml LB, grown ON at 37°C 220 rpm. Tomorrow we will prepare competent cells!! |
Inoculum of: | Inoculum of: | ||
| Line 161: | Line 165: | ||
Cultures were grown ON at 37°C 220 rpm. | Cultures were grown ON at 37°C 220 rpm. | ||
| - | Tomorrow we will test the ability of these | + | Tomorrow we will test the ability of these strains to produce GFP and to lyse when HSL is added. |
| + | |||
| + | ---- | ||
| + | In order to excise the chloramphenicol resistance cassette from the integrants in E.coli genome we transformed the cells with a plasmid (pCP20 plasmid) carrying the gene encoding for the flippase protein. This protein is able to cut fragment between two FRT flanking sequences. pCP20 was transformed in MC43-a and MG43-a and plated on LB+Amp agar plate. | ||
<div align="right"><small>[[#indice|^top]]</small></div> | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==October, 1st== | ==October, 1st== | ||
| + | |||
| + | <font size=4>[[Team:UNIPV-Pavia/Material Methods/Measurements/Tecan/test1ottobre|Tecan Test]]</font> | ||
| + | |||
| + | ---- | ||
This morning, we checked tranformed plates: | This morning, we checked tranformed plates: | ||
| Line 203: | Line 214: | ||
Gel run/cut/extraction/quantification: | Gel run/cut/extraction/quantification: | ||
| + | {|align="center" | ||
| + | |[[Image:UNIPV10_01_10_2010_digestioni_medio.jpg|thumb|200px|center|Intein, I31, I35, I37, I57, I60 digestions]] || [[Image:UNIPV10_01_10_2010_digestioni_psb1c3.jpg|thumb|150px|center|<partinfo>pSB1C3</partinfo> digested E-P (both vector and insert were kept), E-S, X-P.]] | ||
| + | |} | ||
| - | + | *I37 (E-X): 20,4 ng/ul | |
| - | + | *I57 (E-X): 17,6 ng/ul | |
| - | *I37 (E-X): | + | *I60 (E-S): 9,5 ng/ul |
| - | *I57 (E-X): | + | |
| - | *I60 (E-S): | + | |
They were stored at -20°C. | They were stored at -20°C. | ||
| - | *I31 (E-S): | + | *I31 (E-S): 5,4 ng/ul |
| - | *I35 (E-P): | + | *I35 (E-P): 1,0 ng/ul ;-( |
| - | *I35 (E-X): | + | *I35 (E-X): 14,4 ng/ul |
| - | *<partinfo>pSB1C3</partinfo> (E-P): | + | *<partinfo>pSB1C3</partinfo> (E-P): 14,3 ng/ul |
| - | *<partinfo>pSB1C3</partinfo> (E-S): | + | *<partinfo>pSB1C3</partinfo> (E-S): 15,2 ng/ul |
| - | *<partinfo>pSB1C3</partinfo> (X-P): | + | *<partinfo>pSB1C3</partinfo> (X-P): 13,9 ng/ul |
ON ligation of: | ON ligation of: | ||
| Line 221: | Line 233: | ||
*INTEIN_C3: INTEIN (E-P) + <partinfo>pSB1C3</partinfo> (E-P) | *INTEIN_C3: INTEIN (E-P) + <partinfo>pSB1C3</partinfo> (E-P) | ||
| + | ---- | ||
| + | Single colony was picked from MC43 and MG43 plates and inoculated in 5 ml of LB and incubated at 37°C, 220 rpm ON. | ||
| + | 12 hours late 5 ul were streaked on non selective LB plates and incubated at 43°C ON in order to ensure the loss of pCP20 plasmid. | ||
| Line 228: | Line 243: | ||
==October, 2nd== | ==October, 2nd== | ||
| - | PCR from colony for I55 ( | + | PCR from colony for I55 (eleven colonies were picked). |
Gel run: | Gel run: | ||
| - | + | [[Image:UNIPV10_02_10_2010_I55_screening.jpg|thumb|200px|center|I55 colony PCR screening.]] | |
| - | + | ||
| - | + | ||
| + | We decided to make glycerol stock for I55-1. Inoculum into 5 ml LB+Amp of the positive culture that was let grow ON at 37°C, 220 rpm. | ||
---- | ---- | ||
Miniprep and quantification for I73-1/2/3/4/5/6: | Miniprep and quantification for I73-1/2/3/4/5/6: | ||
| - | *I73-1: | + | *I73-1: 154 ng/ul |
| - | *I73-2: | + | *I73-2: 177 ng/ul |
| - | *I73-3: | + | *I73-3: 154,7 ng/ul |
| - | *I73-4: | + | *I73-4: 145,5ng/ul |
| - | *I73-5: | + | *I73-5: 152,6 ng/ul |
| - | *I73-6: | + | *I73-6: 154,2 ng/ul |
| - | 1,5 hours digest (screening) and gel run | + | 1,5 hours E-P digest (for screening) and gel run |
| - | + | [[Image:UNIPV10_02_10_2010_I73_screening.jpg|thumb|200px|center|I70, I71, I73, A9_4C5, A99_4C5 screening.]] | |
| - | As you can see. | + | As you can see they are (except for I73-6) all positive. So we stocked I73-1 and stored it at -80°C. |
---- | ---- | ||
| - | Transformation of I74 and INTEIN_C3 (1ul) in ''E. coli'' TOP10. | + | Transformation of I74 and INTEIN_C3 (1ul) in ''E. coli'' TOP10. They were plated on LB+Amp100 and LB+Cm34 agar plates respectively. They were let grow ON at +37°C. |
| + | ---- | ||
| + | Inoculum of I55-1 into 5 ml LB+Amp. ON growth at +37°C, 220 rpm. | ||
| + | ---- | ||
| + | |||
| + | 3 colonies each plate were picked from MC43_flip and MG43_flip plates and inoculated in 5 ml of non selective LB. To confirm the loss of the chloramphenicol resistance cassette and of the pCP20 plasmid, each colony was inoculated also in 5 ml of LB+Cm12.5 and 5 ml of LB+Amp. | ||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
| + | |||
| + | ==October, 3rd== | ||
| + | Glycerol stock for: | ||
| + | *I55-1 | ||
| + | *IC-1/2/3/4-4C5 | ||
| + | |||
| + | ---- | ||
| + | Pick of a single non-red colony of INTEIN_C3 and inoculum into 5 ml LB+Cm34. Sample was let grow ON at +37°C, 220 rpm. | ||
| + | |||
| + | ---- | ||
| + | Miniprep and quantification of: | ||
| + | *I55-1: 57,4 ng/ul | ||
| + | *I55-1bis: 79,6 ng/ul | ||
| + | |||
| + | I55-1bis was digested E-S for about three hours and than gel run/cut. | ||
| + | |||
| + | ---- | ||
| + | |||
| + | Colony PCR for I74-1..6. | ||
| + | |||
| + | ---- | ||
| + | Gel run for PCR samples and I55 (E-S) and cut for this one. | ||
| + | [[Image:UNIPV10_03_10_2010_I74_screen_I55_cut.jpg|thumb|200px|center|I74 screening and I55 E-S cut.]] | ||
| + | Very bad PCR. We performed a massive PCR from colony during the night (I74-7..24), we ran it the following day. | ||
| + | |||
| + | Gel extraction and quantification: | ||
| + | *I55 (E-S): 5,0 ng/ul | ||
| + | |||
| + | Ligation of I55 (E-S) with already available DNA: | ||
| + | *I75: I55 (E-S) + I37 (E-X) | ||
| + | *I77: I55 (E-S) + I57 (E-X) | ||
| + | ---- | ||
| + | Inoculum of I35 (last quantification after gel extraction was too poor; we will digest it again E-P) and I54 into 5 ml LB+Amp: ON growth at +37°C, 220 rpm. | ||
| + | |||
| + | ---- | ||
| + | Each culture of MC43_flip and MG43_flip in selective LB didn't grown, confirming the loss of pCP20 plasmid and of the chloramphenicol resistance cassette integrated into the E.coli genome. | ||
<div align="right"><small>[[#indice|^top]]</small></div> | <div align="right"><small>[[#indice|^top]]</small></div> | ||
Latest revision as of 18:36, 27 October 2010
|
|
||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||
 "
"