Team:SDU-Denmark/labnotes10
From 2010.igem.org
(Difference between revisions)
(→Lab notes (9/13 - 9/19)) |
(→Insertion of PS + double terminator in pSB3T5 with J13002 (no.2)) |
||
| (8 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
= Lab notes (9/13 - 9/19) = | = Lab notes (9/13 - 9/19) = | ||
__TOC__ | __TOC__ | ||
| + | == Photosensor group == | ||
| + | === Control restriction digest of PS+B0015 in pSB1AK3 sample Q, miniprep === | ||
| + | '''Date:''' 09/14<br> | ||
| + | '''Done by:''' LC<br> | ||
| + | '''Methods:''' Restriction digest<br> | ||
| + | '''Protocols:''' RD1.1[https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1]<br> | ||
| + | '''Notes:''' <br> | ||
| + | The afore mentioned miniprep of PS+B0015 in pSB1AK3 (Q) was cut with EcoRI and PstI to check if biobrick pre- and suffix are present and the insert is the right size. <br> | ||
| + | Mix for 1 RD reaction:<br> | ||
| + | 12 ul H2O<br> | ||
| + | 1 ul PstI<br> | ||
| + | 1 ul EcoRI <bR> | ||
| + | 2 ul Fast digest green buffer<br> | ||
| + | 5 ul PCR product<br> | ||
| + | <br> | ||
| + | Uncut PCR product was loaded on the gel as a control.<br> | ||
| + | <br> | ||
| + | Results: The RD resulted in three bands, uncut plasmid, plasmid and insert. All of those were the expected lengths, so the sample should be OK. <br> | ||
| + | [[Image:Team-SDU-Denmark-PSTQ.jpg|400px]]<br> | ||
| + | |||
| + | === Taq PCR of miniprep of pSB1C3 w. PS col. B and pSB1AK3 w. PS + double termiantor col. C === | ||
| + | '''Date:''' 9/15 2010<Br> | ||
| + | '''Done By:''' Maria and Lc<Br> | ||
| + | '''Protocol:'''[https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.3 CP1.3]<br> | ||
| + | '''Notes:'''<br> | ||
| + | To ensure that the plasminds sent to sequencing contains the correct inserts, af controle PCR of the minipreps using the PS forward and reverse primer is carried out.<br> | ||
| + | Premix x 3<br> | ||
| + | 7.5uL Taq buffer<br> | ||
| + | 3uL MgCl2<br> | ||
| + | 3uL PS FW<br> | ||
| + | 3uL PS RV<br> | ||
| + | 1.5uL dNTP<br> | ||
| + | 52.2uL H2O<br> | ||
| + | 1uL of template is distrubuted into each PCR tube.<br> | ||
| + | PCR program:<br> | ||
| + | <table style="text-align: left;" border="1" cellpadding="2" | ||
| + | cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Start<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">94 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Denaturing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">94 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Annealing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">55 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Elongation<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">72 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2:30 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Goto2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">rep<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">29x<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">End<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">72 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">5 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Hold<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">4 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;"><br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table><br> | ||
| + | The PCR product was loaded onto a 1.5% agarose gel. Gene ruler DNA ladder mix was used as marker.<br><br> | ||
| + | '''Results:'''<br><br> | ||
| + | '''Analysis:'''<br> | ||
| + | 2 bands at app. 3000bp and 4500bp respectively, corresponds to the template sizes, indicating that too much template has been used.<br> | ||
| + | --[[User:Tipi|Tipi]] 06:44, 28 September 2010 (UTC)<br><br> | ||
| + | |||
=== Insertion of PS + double terminator in pSB3T5 with J13002 === | === Insertion of PS + double terminator in pSB3T5 with J13002 === | ||
| Line 269: | Line 369: | ||
==== Gel extraction of PS + double terminator ==== | ==== Gel extraction of PS + double terminator ==== | ||
| - | '''Date:''' 9/ | + | '''Date:''' 9/14 2010<Br> |
'''Done By:''' Maria and Lc<Br> | '''Done By:''' Maria and Lc<Br> | ||
'''Protocol:'''[https://2010.igem.org/Team:SDU-Denmark/protocols#DE1.3 DE1.3]<br> | '''Protocol:'''[https://2010.igem.org/Team:SDU-Denmark/protocols#DE1.3 DE1.3]<br> | ||
| Line 278: | Line 378: | ||
==== Restriction digest of PS + double terminator, and PSB3T5 (no.2) ==== | ==== Restriction digest of PS + double terminator, and PSB3T5 (no.2) ==== | ||
| - | '''Date:''' 9/ | + | '''Date:''' 9/14 2010<Br> |
'''Done By:''' Maria and Lc<Br> | '''Done By:''' Maria and Lc<Br> | ||
'''Protocol:'''[https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1 RD1.1][https://2010.igem.org/Team:SDU-Denmark/protocols#DE1.3 DE1.3]<br> | '''Protocol:'''[https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1 RD1.1][https://2010.igem.org/Team:SDU-Denmark/protocols#DE1.3 DE1.3]<br> | ||
| Line 342: | Line 442: | ||
<tr> | <tr> | ||
<td>1</td> | <td>1</td> | ||
| - | <td> | + | <td>Marker</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 350: | Line 450: | ||
<tr> | <tr> | ||
<td>3</td> | <td>3</td> | ||
| - | <td> | + | <td>PS + double terminator</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| Line 358: | Line 458: | ||
<tr> | <tr> | ||
<td>5</td> | <td>5</td> | ||
| - | <td> | + | <td>pSB3T5</td> |
</tr> | </tr> | ||
</table><br> | </table><br> | ||
| - | DNA was extracted from gel, | + | DNA was extracted from gel, according to protocol. Samples were diluted in 20uL H2O. 2. dilution of PS + doubletermiantor was diluted in 10uL H2O<br><br> |
'''Results:'''<br> | '''Results:'''<br> | ||
DNA conc: | DNA conc: | ||
| Line 371: | Line 471: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>PS ( | + | <td>PS + double terminator (1. dilution)</td> |
| - | <td> | + | <td>4.8</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>PS ( | + | <td>PS + double terminator (2. dilution)</td> |
| - | <td> | + | <td>2.3</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td> | + | <td>pSB3T5</td> |
| - | <td> | + | <td>26.4</td> |
</tr> | </tr> | ||
| - | + | </table> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
'''Analysis:''' | '''Analysis:''' | ||
the purified DNA was used for ligation.<br><br> | the purified DNA was used for ligation.<br><br> | ||
| - | ==== Ligation of PS and | + | ==== Ligation of PS + double terminator and pSB3T5 ==== |
| - | '''Date:''' 9/ | + | '''Date:''' 9/14 2010<Br> |
'''Done By:''' Maria and Lc<Br> | '''Done By:''' Maria and Lc<Br> | ||
'''Protocol:'''[https://2010.igem.org/Team:SDU-Denmark/protocols#LG1.2 LG1.2]<br> | '''Protocol:'''[https://2010.igem.org/Team:SDU-Denmark/protocols#LG1.2 LG1.2]<br> | ||
'''Notes:'''<br> | '''Notes:'''<br> | ||
| - | + | Three ligation mixtures was prepared. vector concentrations of 25ng/uL was used for each mixture. Appropiate amount of insert was added to reac vector:insert ratios of 1:1, 1:2 and 1:3 respectively. <br> | |
| - | Ligation mixtures (PS in | + | Ligation mixtures (PS + double terminator in pSB3T5):<br> |
<table style="text-align: left;" border="1" | <table style="text-align: left;" border="1" | ||
cellpadding="2" cellspacing="2"> | cellpadding="2" cellspacing="2"> | ||
| Line 418: | Line 515: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td> | + | <td>pSB3T5</td> |
| - | <td> | + | <td>1uL</td> |
| - | <td> | + | <td>1uL</td> |
| - | <td> | + | <td>1uL</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>PS </td> | + | <td>PS + double terminator </td> |
| - | <td> | + | <td>8uL (2. dilution)</td> |
| - | <td> | + | <td>7uL</td> |
| - | <td> | + | <td>11uL</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
<td>H<small>2</small>0</td> | <td>H<small>2</small>0</td> | ||
| + | <td>8uL</td> | ||
| + | <td>9uL</td> | ||
| + | <td>5uL</td> | ||
| + | </tr> | ||
| + | </table><br> | ||
| + | |||
| + | The samples were incubated at 17C ON at used for transformation<br><br> | ||
| + | |||
| + | ==== Transfomation of ligated plasmid in Top 10 E.coli ==== | ||
| + | |||
| + | '''Date:''' 9/15 2010<Br> | ||
| + | '''Done By:''' Maria and Lc<Br> | ||
| + | '''Protocol:'''[https://2010.igem.org/Team:SDU-Denmark/protocols#CC1.1 CC1.1][https://2010.igem.org/Team:SDU-Denmark/protocols#TR1.1 TR1.1]<br> | ||
| + | '''Notes:'''<br> | ||
| + | The compotent cells and transformation was carried out according to protocol.<br><br> | ||
| + | '''Results:'''<br> | ||
| + | the controle plates were okay, and colonies of different sizes was observed on plates of cells transformed with ligations.<br><br> | ||
| + | '''Analysis:'''<br> | ||
| + | The transformation was successfull and colonies was selected and used in coloni PCR.<br> | ||
| + | --[[User:Tipi|Tipi]] 13:41, 26 September 2010 (UTC)<br><br> | ||
| + | === Transfomation of pKJ606 in Mg 1655 E.coli === | ||
| + | |||
| + | '''Date:''' 9/15 2010<Br> | ||
| + | '''Done By:''' Maria and Lc<Br> | ||
| + | '''Protocol:'''[https://2010.igem.org/Team:SDU-Denmark/protocols#CC1.1 CC1.1][https://2010.igem.org/Team:SDU-Denmark/protocols#TR1.1 TR1.1]<br> | ||
| + | '''Notes:'''<br> | ||
| + | pKJ606 plasmid was transformed into Mg1655, to use for characterization. <br>The compotent cells and transformation was carried out according to protocol.<br><br> | ||
| + | '''Results:'''<br> | ||
| + | the controle plates were okay, and large colonies was observed on the plates.<br><br> | ||
| + | '''Analysis:'''<br> | ||
| + | The transformation was successfull and colonies was selected and used in coloni PCR.<br> | ||
| + | --[[User:Tipi|Tipi]] 05:48, 28 September 2010 (UTC)<br><br> | ||
| + | |||
| + | === Colony PCR of transformation of pKJ606 in Mg1655 and pSB3T5 w. PS + double terminator in top10 (no.1)=== | ||
| + | '''Date:''' 09/16<br> | ||
| + | '''Done by:''' LC & Maria<br> | ||
| + | '''Methods:''' Colony PCR<br> | ||
| + | '''Protocols:''' CP1.3[https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.3]<br> | ||
| + | '''Notes:''' <br> | ||
| + | for the pKJ606 coloni PCR two colonies are selected.<br> | ||
| + | 16 colonies of different sizes are selected from the ligation plates. The sizes of the colonies are noted down to test whether this can be used to select colonies containing the correct plasmid<br> | ||
| + | <table style="text-align: left;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td>coloni sizes</td> | ||
| + | <td>large </td> | ||
| + | <td>small</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>colonies</td> | ||
| + | <td>K,B,A,C,D,E,F,I,J,N,O,P</td> | ||
| + | <td>L,G,H,M</td> | ||
| + | </tr> | ||
| + | </table><br><br> | ||
| + | |||
| + | Premix for pKJ606 (x3) :<br> | ||
| + | 7.5 µl 10xTAQ Buffer<br> | ||
| + | 3 µl MgCl2 <br> | ||
| + | 3 µl FW PS <br> | ||
| + | 3 µl PV PS <br> | ||
| + | 1.5 µl dNTP<br> | ||
| + | 10.5 µl H2O<br> | ||
| + | 1 µl TAQ Polymerase<br><br> | ||
| + | |||
| + | Premix for pSB3T5 (x17) :<br> | ||
| + | 42.5 µl 10xTAQ Buffer<br> | ||
| + | 17 µl MgCl2 <br> | ||
| + | 17 µl VF2 <br> | ||
| + | 17 µl VR <br> | ||
| + | 8.5 µl dNTP<br> | ||
| + | 59.5 µl H2O<br> | ||
| + | 2 µl TAQ Polymerase<br><br> | ||
| + | The chosen colonies were lysed in 15 ul of H20.<br> | ||
| + | Both of the coloni PCR's were run using the same program. | ||
| + | <br> | ||
| + | PCR Program:<br> | ||
| + | <table style="text-align: left;" border="1" cellpadding="2" | ||
| + | cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Start<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">94 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Denaturing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">94 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Annealing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">55 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Elongation<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">72 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2:30 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Goto2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">rep<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">29x<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">End<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">72 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">5 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Hold<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">4 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;"><br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table><br> | ||
| + | PCR product was loaded onto a 1.5% agarose gel. Gene ruler DNA ladder mix was used as marker.<br><br> | ||
| + | '''Results:'''<br> | ||
| + | [[Image:Team-SDU-Denmark-CPCRF1.jpg|400px]]<br> | ||
| + | '''Analysis:'''<br> | ||
| + | No bands was obeserved on the gel, and the experiment was redone.<br> | ||
| + | --[[User:Tipi|Tipi]] 06:25, 28 September 2010 (UTC)<br><br> | ||
| + | |||
| + | === Colony PCR of transformation of pKJ606 in Mg1655 and pSB3T5 w. PS + double terminator in top10 (no.2)=== | ||
| + | '''Date:''' 09/16<br> | ||
| + | '''Done by:''' LC & Maria<br> | ||
| + | '''Methods:''' Colony PCR<br> | ||
| + | '''Protocols:''' CP1.3[https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.3]<br> | ||
| + | '''Notes:''' <br> | ||
| + | for the pKJ606 coloni PCR two colonies are selected.<br> | ||
| + | 16 colonies of different sizes are selected from the ligation plates. The sizes of the colonies are noted down to test whether this can be used to select colonies containing the correct plasmid<br> | ||
| + | <table style="text-align: left;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td>coloni sizes</td> | ||
| + | <td>large </td> | ||
| + | <td>small</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>colonies</td> | ||
| + | <td>O,L,G,A,B</td> | ||
| + | <td>P,N,K,I,H,E,M,J,F,D</td> | ||
| + | </tr> | ||
| + | </table><br><br> | ||
| + | |||
| + | Premix for pKJ606 (x3) :<br> | ||
| + | 7.5 µl 10xTAQ Buffer<br> | ||
| + | 3 µl MgCl2 <br> | ||
| + | 3 µl FW PS <br> | ||
| + | 3 µl PV PS <br> | ||
| + | 1.5 µl dNTP<br> | ||
| + | 10.5 µl H2O<br> | ||
| + | 1 µl TAQ Polymerase<br><br> | ||
| + | |||
| + | Premix for pSB3T5 (x17) :<br> | ||
| + | 42.5 µl 10xTAQ Buffer<br> | ||
| + | 17 µl MgCl2 <br> | ||
| + | 17 µl VF2 <br> | ||
| + | 17 µl VR <br> | ||
| + | 8.5 µl dNTP<br> | ||
| + | 59.5 µl H2O<br> | ||
| + | 2 µl TAQ Polymerase<br><br> | ||
| + | The chosen colonies were lysed in 15 ul of H20.<br> | ||
| + | Both of the coloni PCR's were run using the same program. | ||
| + | <br> | ||
| + | PCR Program:<br> | ||
| + | <table style="text-align: left;" border="1" cellpadding="2" | ||
| + | cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Start<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">94 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Denaturing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">94 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Annealing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">55 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Elongation<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">72 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2:30 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Goto2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">rep<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">29x<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">End<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">72 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">5 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Hold<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">4 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;"><br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table><br> | ||
| + | PCR product was loaded onto a 1.5% agarose gel. Gene ruler DNA ladder mix was used as marker.<br><br> | ||
| + | '''Results:'''<br><br> | ||
| + | '''Analysis:'''<br> | ||
| + | In the lanes containing PCR product of colonies transformed with pKJ606 bands at app. 2000bp are observed, indicating that the colonies contains the right plasmids. No bands was observed in lanes containing PCR product of colonies transformed with pSB3T5, and this experiment needs to be redone.<br> | ||
| + | --[[User:Tipi|Tipi]] 06:25, 28 September 2010 (UTC)<br><br> | ||
| + | |||
| + | === Insertion of PS + double terminator in pSB3T5 with J13002 (no.2) === | ||
| + | |||
| + | '''Date:''' 9/16 - 9/19 2010<Br> | ||
| + | '''Done By:''' Maria and Lc<Br> | ||
| + | '''Protocol:''' [https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1 CP1.1][https://2010.igem.org/Team:SDU-Denmark/protocols#DE1.3 DE1.3][https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1 RD1.1][https://2010.igem.org/Team:SDU-Denmark/protocols#LG1.2 LG1.2][https://2010.igem.org/Team:SDU-Denmark/protocols#CC1.1 CC1.1][https://2010.igem.org/Team:SDU-Denmark/protocols#TR1.1 TR1.1][https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.3 CP1.3]<Br> | ||
| + | |||
| + | ==== PFU PCR on PS+B0015 with VF2 and VR primers ==== | ||
| + | '''Date:''' 9/16 - 9/19 2010<Br> | ||
| + | '''Done By:''' Maria and LC<Br> | ||
| + | '''Protocol:''' [https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.3 CP1.3]<br> | ||
| + | '''Notes:'''<br> | ||
| + | <br> | ||
| + | Premix:<br> | ||
| + | 30 ul PFU Buffer + MgSO4<br> | ||
| + | 9 ul dNTP mix <br> | ||
| + | 9 ul VF2 <br> | ||
| + | 9 ul VR <br> | ||
| + | 10 ul template (5 ul of PS in pSB1AK3 diluted in 5 ul H2O)<br> | ||
| + | 230 ul H2O <br> | ||
| + | 2,5 ul PFU polymerase<br> | ||
| + | <br> | ||
| + | Program:<br> | ||
| + | <table style="text-align: left;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td>start</td> | ||
| + | <td>95C</td> | ||
| + | <td>3min</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>denaturating</td> | ||
| + | <td>95C</td> | ||
| + | <td>2min</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>annealing</td> | ||
| + | <td>55C</td> | ||
| + | <td>30s</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>elongation</td> | ||
| + | <td>72C</td> | ||
| + | <td>5 min</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>go to</td> | ||
| + | <td>2</td> | ||
| + | <td>rep.29x</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>end</td> | ||
| + | <td>72C</td> | ||
| + | <td>5min</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>hold</td> | ||
| + | <td>4C</td> | ||
| + | <td></td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <br> | ||
| + | '''Results:''' The band showed up at the correct length as expected. The remaining product was then extracted from the PCR mixture.<br> | ||
| + | |||
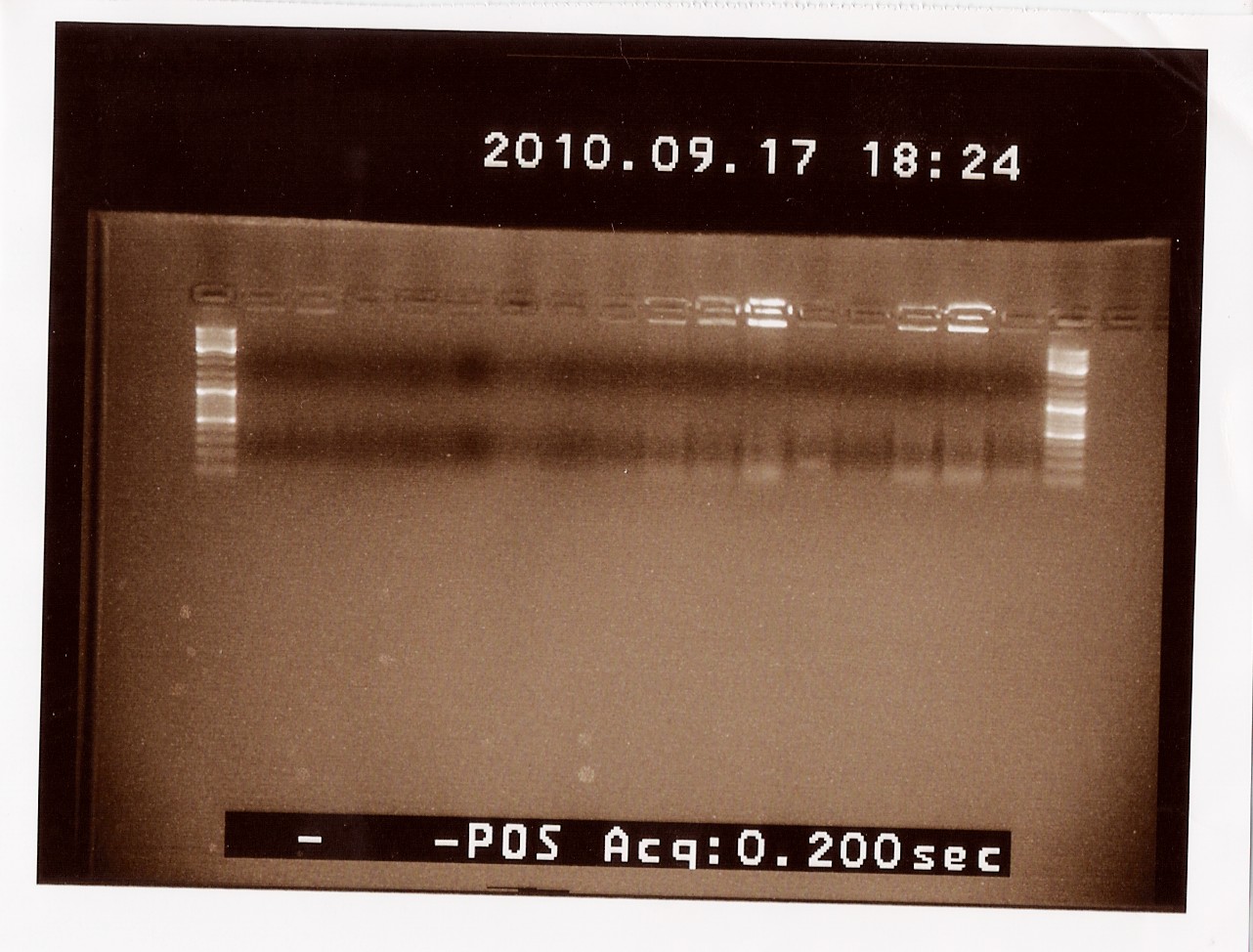
| + | ==== Restriction digest of PS + double terminator and PSB3T5 ==== | ||
| + | '''Date:''' 9/17 2010<Br> | ||
| + | '''Done By:''' Maria and Lc<Br> | ||
| + | '''Protocol:'''[https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1 RD1.1][https://2010.igem.org/Team:SDU-Denmark/protocols#DE1.3 DE1.3]<br> | ||
| + | '''Notes:'''<br> | ||
| + | Restriction mixture pSB3T5:<br> | ||
| + | <table style="text-align: left;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td>H<small>2</small>O</td> | ||
<td>14uL</td> | <td>14uL</td> | ||
| - | <td> | + | </tr> |
| - | <td> | + | <tr> |
| + | <td>FD green buffer</td> | ||
| + | <td>4uL</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>SpeI</td> | ||
| + | <td>2uL</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>PstI</td> | ||
| + | <td>2uL</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pSB3T5</td> | ||
| + | <td>20uL</td> | ||
| + | </tr> | ||
| + | </table><br><br> | ||
| + | |||
| + | Restriction mixture PS + double terminator:<br> | ||
| + | <table style="text-align: left;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td>H<small>2</small>O</td> | ||
| + | <td>38uL</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>FD green buffer</td> | ||
| + | <td>8uL</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>XbaI</td> | ||
| + | <td>4uL</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>PstI</td> | ||
| + | <td>4uL</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>PS + double terminator</td> | ||
| + | <td>30uL</td> | ||
| + | </tr> | ||
| + | </table><br><br> | ||
| + | Samples were digested for 10min. at 37C.<br> | ||
| + | Digested samples were loaded onto a 1.5% agarose extraction gel. Uncut PS + double terminator and pSB3T5 were used as controles. Gene ruler DNA ladder mix was used as marker.<br> | ||
| + | Loading scheme:<br> | ||
| + | <table style="text-align: left;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td>Lane</td> | ||
| + | <td>sample</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>1</td> | ||
| + | <td>Marker</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>2</td> | ||
| + | <td>pSB3T5</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>3</td> | ||
| + | <td>uncut pSB3T5</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>4</td> | ||
| + | <td>PS + double terminator</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>5</td> | ||
| + | <td>uncut PS + double terminator</td> | ||
</tr> | </tr> | ||
</table><br> | </table><br> | ||
| - | Ligation mixtures (PS in | + | DNA was extracted from gel, according to protocol. Samples were diluted in 20uL H2O.<br><br> |
| + | '''Results:'''<br> | ||
| + | DNA conc: | ||
| + | <table style="text-align: left;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td>sample</td> | ||
| + | <td>conc. (ng/uL)</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>PS + double terminator</td> | ||
| + | <td>26.3</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>pSB3T5</td> | ||
| + | <td>25.8</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
| + | '''Analysis:''' | ||
| + | the purified DNA was used for ligation.<br><br> | ||
| + | |||
| + | ==== Ligation of PS + double terminator and pSB3T5 ==== | ||
| + | '''Date:''' 9/17 2010<Br> | ||
| + | '''Done By:''' Maria and Lc<Br> | ||
| + | '''Protocol:'''[https://2010.igem.org/Team:SDU-Denmark/protocols#LG1.2 LG1.2]<br> | ||
| + | '''Notes:'''<br> | ||
| + | Three ligation mixtures was prepared. 50ng of vector was used for each mixture. Appropiate amount of insert was added to reac vector:insert ratios of 1:1, 1:2 and 1:4 respectively. <br> | ||
| + | Ligation mixtures (PS + double terminator in pSB3T5):<br> | ||
<table style="text-align: left;" border="1" | <table style="text-align: left;" border="1" | ||
cellpadding="2" cellspacing="2"> | cellpadding="2" cellspacing="2"> | ||
| Line 458: | Line 975: | ||
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td> | + | <td>pSB3T5</td> |
| - | <td> | + | <td>2uL</td> |
| - | <td> | + | <td>2uL</td> |
| - | <td> | + | <td>2uL</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
| - | <td>PS </td> | + | <td>PS + double terminator </td> |
| - | <td> | + | <td>1uL (2. dilution)</td> |
| - | <td> | + | <td>2.5uL</td> |
| - | <td> | + | <td>5uL</td> |
</tr> | </tr> | ||
<tr> | <tr> | ||
<td>H<small>2</small>0</td> | <td>H<small>2</small>0</td> | ||
<td>14uL</td> | <td>14uL</td> | ||
| - | <td> | + | <td>12.5uL</td> |
| - | <td> | + | <td>10uL</td> |
</tr> | </tr> | ||
| - | </table | + | </table><br> |
| - | The samples | + | |
| + | The samples were incubated at 17C ON at used for transformation<br><br> | ||
==== Transfomation of ligated plasmid in Top 10 E.coli ==== | ==== Transfomation of ligated plasmid in Top 10 E.coli ==== | ||
| - | '''Date:''' 9/ | + | '''Date:''' 9/15 2010<Br> |
'''Done By:''' Maria and Lc<Br> | '''Done By:''' Maria and Lc<Br> | ||
'''Protocol:'''[https://2010.igem.org/Team:SDU-Denmark/protocols#CC1.1 CC1.1][https://2010.igem.org/Team:SDU-Denmark/protocols#TR1.1 TR1.1]<br> | '''Protocol:'''[https://2010.igem.org/Team:SDU-Denmark/protocols#CC1.1 CC1.1][https://2010.igem.org/Team:SDU-Denmark/protocols#TR1.1 TR1.1]<br> | ||
'''Notes:'''<br> | '''Notes:'''<br> | ||
| - | The compotent cells and transformation was carried out according to protocol | + | The compotent cells and transformation was carried out according to protocol.<br><br> |
'''Results:'''<br> | '''Results:'''<br> | ||
| - | the controle plates were okay, and | + | the controle plates were okay, and colonies of different sizes was observed on plates of cells transformed with ligations.<br><br> |
'''Analysis:'''<br> | '''Analysis:'''<br> | ||
The transformation was successfull and colonies was selected and used in coloni PCR.<br> | The transformation was successfull and colonies was selected and used in coloni PCR.<br> | ||
| - | --[[User:Tipi|Tipi]] | + | --[[User:Tipi|Tipi]] 11:21, 28 September 2010 (UTC)<br><br> |
| + | |||
| + | |||
</div> | </div> | ||
Latest revision as of 09:36, 30 September 2010
 "
"