Team:SDU-Denmark/labnotes6
From 2010.igem.org
(Difference between revisions)
(→Group: Retinal) |
(→Lab notes (16/9 - 22/9)) |
||
| (6 intermediate revisions not shown) | |||
| Line 9: | Line 9: | ||
== Group: Retinal == | == Group: Retinal == | ||
| + | ==== ON of Top 10 E. coli cells with POT2 with NinaB ==== | ||
| + | Date: 16/8<br> | ||
| + | Done by: Marie & Tommy<br> | ||
| + | Methods: | ||
| + | protocos: | ||
| + | Notes:<br> | ||
| + | 100 mL ON culture was made, cells were grown at 37 C in LB media with Chloramphenicol. | ||
| + | ==== ON of Top 10 E. coli cells with POT2 with NinaB ==== | ||
| + | Date: 17/8<br> | ||
| + | Done by: Marie & Tommy<br> | ||
| + | Methods: Miniprep, Restriction digest, gel<br> | ||
| + | Protocols: [https://2010.igem.org/Team:SDU-Denmark/protocols#MP1.2 MP1.2], [https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1 RD1.1]. | ||
| + | Notes:<br> | ||
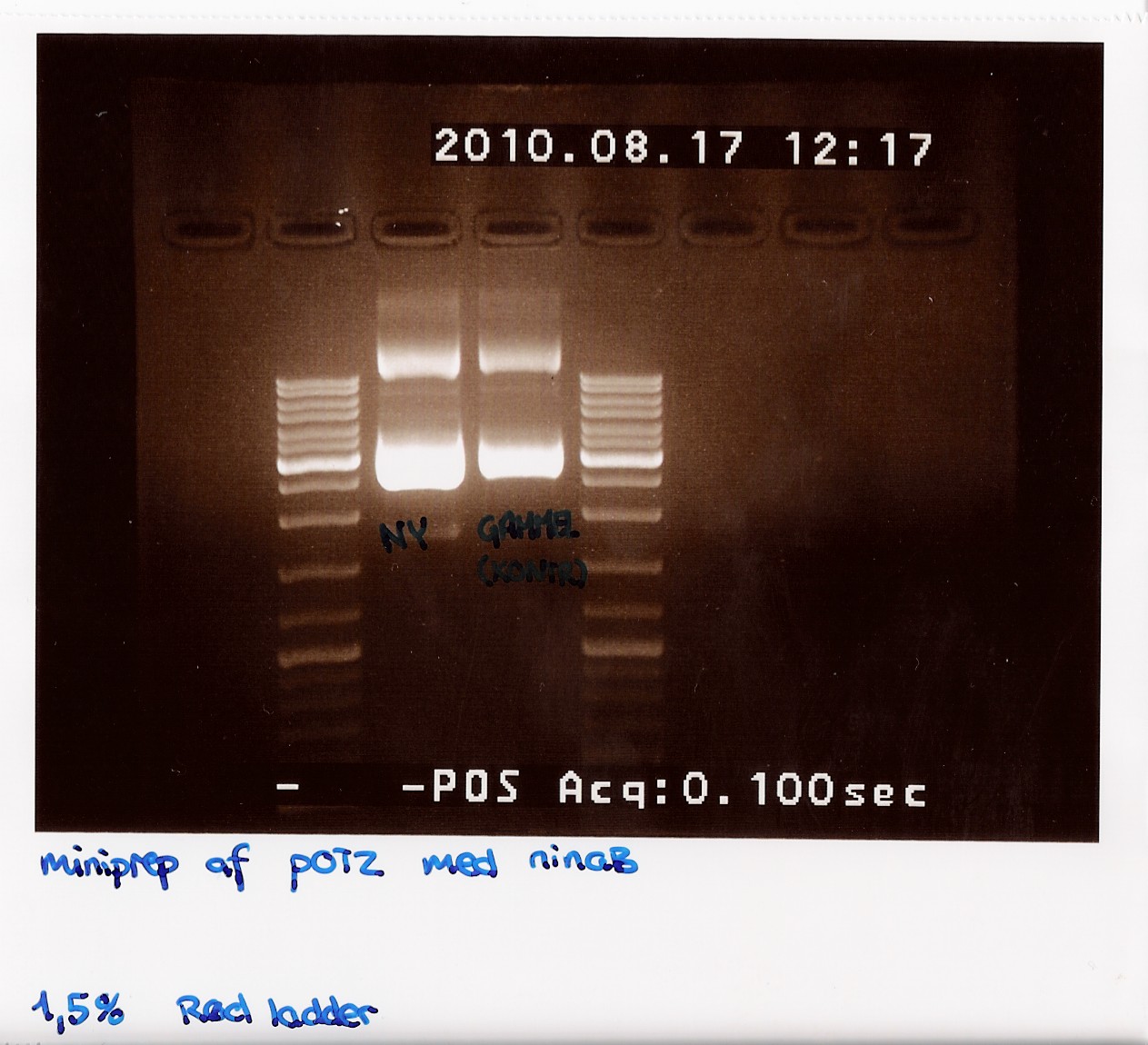
| + | 50 mL of elution buffer was used. DNA concentrations after pooling were measured on NanoDrop to 206,2 ng/microL. | ||
| + | gel was run on produckt, showing recent miniprep compared to old miniprep produckt, showing uneven lengths. subsequent was performed.<br> | ||
| + | [[Image:Team-sdu-denmark-Minipreb_product_of_POT2_with_NinaB_1,5_gel_red_loader__-4.jpg | 300px ]] | ||
| + | ==== Restriction digest on miniprep produckt (w. EcoRI) ==== | ||
| + | Date: 17/8<br> | ||
| + | Done by: Marie & Tommy<br> | ||
| + | Methods: Restriction digest, gel<br> | ||
| + | Protocols: [https://2010.igem.org/Team:SDU-Denmark/protocols#MP1.2 MP1.2], gel. | ||
| + | Notes:<br> | ||
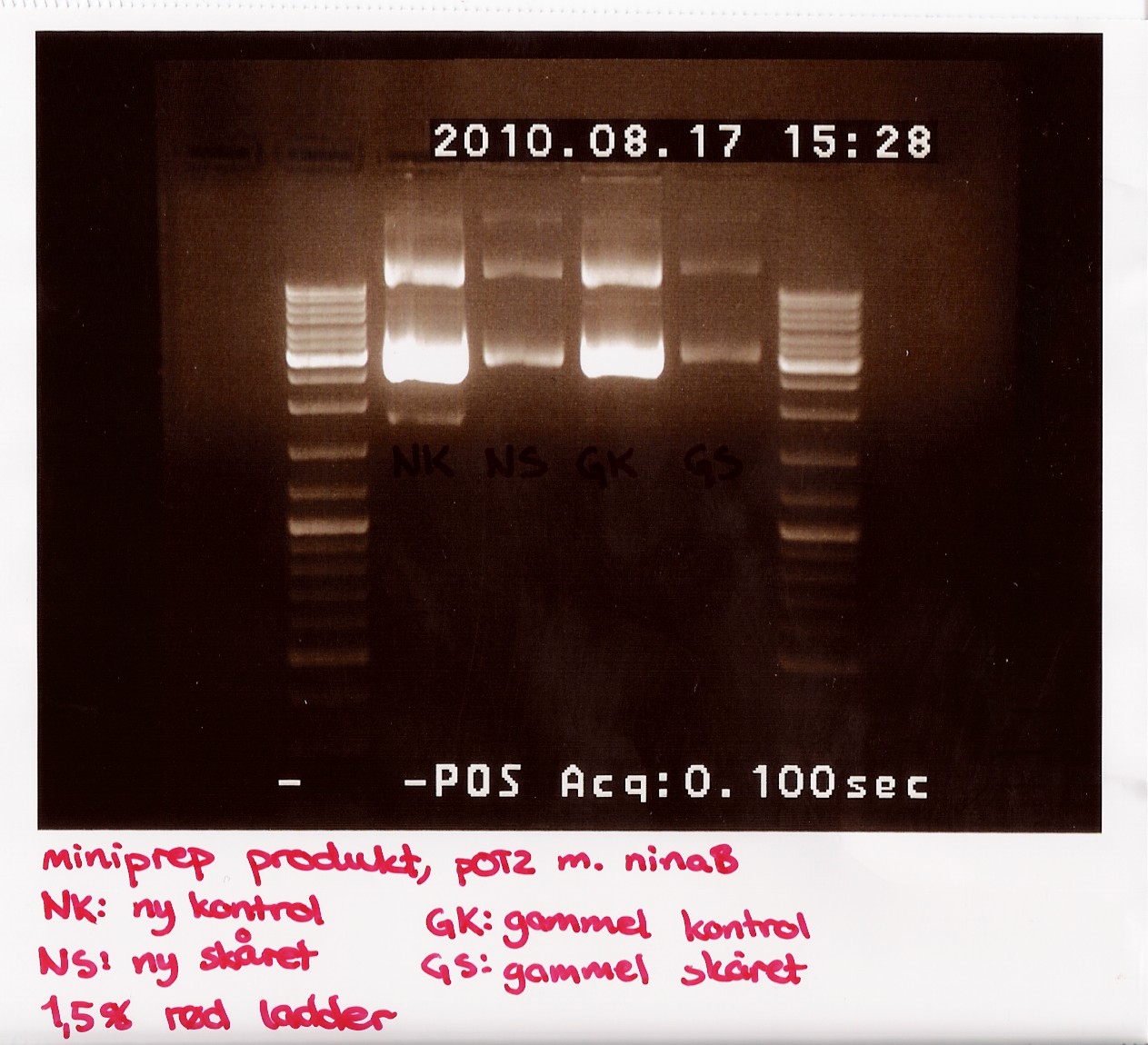
| + | Due to the lack of old sample, restriction digest was performed using only 3,5 microL of miniprep produckt. 1,5 microL of H2O was added insted. EcoRI was used<br> | ||
| + | The digest mix was incubated for 10 min at 37 C. A gel was run showing uncut new, cut new, uncut old and cut old miniprep product.<br> | ||
| + | [[Image:Team-sdu-denmark-Minipreb_product_of_POT2_with_NinaB_1,5_gel_red_loader__-5.jpg |300px ]] | ||
| + | |||
| + | === PCR on POT2 with NinaB (New Primers) === | ||
| + | Start date: 20/8<br> | ||
| + | Methods: PCR, Gel<br> | ||
| + | Protocols: [https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1 CP.1.1] | ||
| + | |||
| + | ==== PCR on POT2 with NinaB (New Primers) ==== | ||
| + | Date: 20/8<br> | ||
| + | Done by: Marie & Tommy<br> | ||
| + | Methods: ON<br> | ||
| + | protocos:[https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1 CP1.1] | ||
| + | Notes:<br> | ||
| + | The new primers only contain the innermost restriction sites.<br> | ||
| + | Meltting temp.: FWD: 68,1 C REV: 65,6 C<br> | ||
| + | To get the optimal PCR temperatures a gradient PCR were run programed as:<br> | ||
| + | <html> | ||
| + | <head> | ||
| + | <meta content="text/html; charset=ISO-8859-1" | ||
| + | http-equiv="content-type"> | ||
| + | <title></title> | ||
| + | </head> | ||
| + | <body> | ||
| + | <table style="text-align: left; width: 300px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td>PCR</td> | ||
| + | <td>Temp. (C)</td> | ||
| + | <td>Time (min)</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Start</td> | ||
| + | <td>95</td> | ||
| + | <td>2</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Denaturing</td> | ||
| + | <td>95</td> | ||
| + | <td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Anneling</td> | ||
| + | <td>Gradient</td> | ||
| + | <td>1</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Elongation</td> | ||
| + | <td>72</td> | ||
| + | <td>4</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>End</td> | ||
| + | <td>72</td> | ||
| + | <td>5</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>Hold</td> | ||
| + | <td>4</td> | ||
| + | <td>indef.</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <br> | ||
| + | </body> | ||
| + | </html> | ||
| + | <br> | ||
| + | The temperatur gradient were run from 60 to 70 C and the samples were run at these temperatures:<br> | ||
| + | <html> | ||
| + | <head> | ||
| + | <meta content="text/html; charset=ISO-8859-1" | ||
| + | http-equiv="content-type"> | ||
| + | <title></title> | ||
| + | </head> | ||
| + | <body> | ||
| + | <table style="text-align: left; width: 300px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td>Sample</td> | ||
| + | <td>Colunm</td> | ||
| + | <td>Temp. C</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>1</td> | ||
| + | <td>1</td> | ||
| + | <td>59,9</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>2</td> | ||
| + | <td>3</td> | ||
| + | <td>60,7</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>3</td> | ||
| + | <td>5</td> | ||
| + | <td>62,7</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>4</td> | ||
| + | <td>7</td> | ||
| + | <td>65,4</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>5</td> | ||
| + | <td>9</td> | ||
| + | <td>67,9</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>6</td> | ||
| + | <td>11</td> | ||
| + | <td>69,6</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <br> | ||
| + | </body> | ||
| + | </html><br> | ||
| + | After the PRC the product were run on a gel<br> | ||
| + | |||
| + | The gel picture shows that alle of the temperatures gave results. | ||
Latest revision as of 22:21, 23 October 2010
 "
"