Team:Lethbridge/Notebook/Lab Work/July
From 2010.igem.org
(→July 5/2010) |
Liszabruder (Talk | contribs) |
||
| (104 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | <div style="background-color:#000000; color:white"> | |
| + | <html> | ||
| - | + | <br> | |
| - | + | <table border="0" width="100%" style="background-color:#000000"> | |
| - | + | <tr> | |
| - | + | <th> | |
| - | + | <image src="https://static.igem.org/mediawiki/2010/2/29/UofLteamlogo.jpg" width="200px"/> | |
| - | + | ||
| - | + | </th> | |
| - | + | ||
| - | + | ||
| - | + | <th> | |
| - | + | ||
| - | + | ||
| - | + | <image src="https://static.igem.org/mediawiki/2010/9/91/UofLLabWork.JPG" height="300px"/> | |
| - | + | ||
| - | + | ||
| - | + | </th> | |
| - | + | ||
| - | + | ||
| - | + | <th> | |
| - | + | ||
| - | + | ||
| - | + | <image src="https://static.igem.org/mediawiki/2010/2/29/UofLteamlogo.jpg" width="200px"/> | |
| - | + | ||
| - | + | ||
| - | + | </th> | |
| - | + | ||
| - | + | ||
| - | + | </tr> | |
| - | + | ||
| - | + | ||
| - | + | </table> | |
| - | + | ||
| - | + | ||
| - | + | <br> | |
| - | =July 2010= | + | |
| - | ==July 5/2010== | + | <align="centre"> |
| + | <table border="0" width="100%" style="background-color:#000000"> | ||
| + | |||
| + | <tr> | ||
| + | |||
| + | <th> | ||
| + | |||
| + | <a href="https://2010.igem.org/Team:Lethbridge"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/2/22/UofLHome.jpg" width="80"/> | ||
| + | </a> | ||
| + | |||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Team"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/0/0d/UofLTeam.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Project"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/8/8d/UofLProjectbutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/7/73/UofLNotebookbutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Parts"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/8/84/UofLPartsSubmittedToTheRegistrybutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Modeling"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/e/e1/UofLModelingbutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Ethics"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/2/26/UofLEthicsbutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Safety"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/0/00/UofLSafetybutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Art"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/0/0a/UofLArt.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/News"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/c/c3/UofLNewsButton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | </table> | ||
| + | </body> | ||
| + | </html> | ||
| + | <hr> | ||
| + | |||
| + | <html> | ||
| + | <center> | ||
| + | <font color="white">Feel free to look around our notebook! | ||
| + | </center> | ||
| + | </html> | ||
| + | |||
| + | <html> | ||
| + | <body> | ||
| + | <center> | ||
| + | <table border="0" width="28%" style="background-color:#000000"> | ||
| + | |||
| + | <tr> | ||
| + | <th> | ||
| + | <div class="miniBar"> | ||
| + | <div class="countdown"><object type="application/x-shockwave-flash" data="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=10&date_day=27&date_year=0&un=THE WIKI FREEZE&size=normal&mo=10&da=27&yr=2010" width="300" height="100"><param name="movie" value="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=10&date_day=27&date_year=0&un=THE WIKI FREEZE&size=normal&mo=10&da=27&yr=2010" /><param name="bgcolor" value="#000000" /></object><img src="http://www.oneplusyou.com/q/img/bb_badges/countdown.jpg" alt="" style="display: none;" height="1" width="1" /></div> | ||
| + | <div class="miniContainer"> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/7/73/UofLNotebookbutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Protocols"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/9/91/UofLprotocolsbutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Calendar"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/7/73/UofLcalendar.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th> | ||
| + | <div class="miniBar"> | ||
| + | <div class="countdown"><object type="application/x-shockwave-flash" data="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=11&date_day=05&date_year=0&un=THE IGEM JAMBOREE&size=normal&mo=11&da=05&yr=2010" width="300" height="100"><param name="movie" value="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=11&date_day=05&date_year=0&un=THE IGEM JAMBOREE&size=normal&mo=11&da=05&yr=2010" /><param name="bgcolor" value="#000000" /></object><img src="http://www.oneplusyou.com/q/img/bb_badges/countdown.jpg" alt="" style="display: none;" height="1" width="1" /></div> | ||
| + | <div class="miniContainer"> | ||
| + | </th> | ||
| + | <tr> | ||
| + | </table> | ||
| + | </center> | ||
| + | </body> | ||
| + | </html> | ||
| + | |||
| + | <hr> | ||
| + | |||
| + | <html> | ||
| + | <center> | ||
| + | <font color="white">Here you can check out the work we have done in the lab, click on a month to take a look! | ||
| + | </center> | ||
| + | </html> | ||
| + | |||
| + | <html> | ||
| + | <body> | ||
| + | <center> | ||
| + | <table border="0" width="50%" style="background-color:#000000"> | ||
| + | |||
| + | <tr> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/April"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/8/8a/UofLapril.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/May"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/7/7b/UofLmaybutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/June"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/8/80/UofLjunebutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/July"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/5/53/UofLjulybutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/August"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/1/15/UofLaugustbutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/September"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/4/4d/UofLseptemberbutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/October"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/4/4e/UofLoctoberbutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | |||
| + | |||
| + | <tr> | ||
| + | </table> | ||
| + | </center> | ||
| + | </body> | ||
| + | </html> | ||
| + | <hr> | ||
| + | |||
| + | <BLOCKQUOTE> | ||
| + | |||
| + | =<font color="white">July 2010= | ||
| + | ==<font color="white">July 3/2010== | ||
| + | (In Lab: JS)<br> | ||
| + | |||
| + | <b>Objective:</b> To restrict and ligate pBAD-TetR and YEPs into pSB1C3 backbone.<br> | ||
| + | |||
| + | <b>Method:</b> Used [[Team:Lethbridge/Notebook/Protocols|Restriction of Plasmid DNA]] protocol and ligated the parts into pSB1C3.<br> | ||
| + | |||
| + | ==<font color="white">July 5/2010== | ||
(In Lab: JV, AV, HB)<br> | (In Lab: JV, AV, HB)<br> | ||
| Line 98: | Line 256: | ||
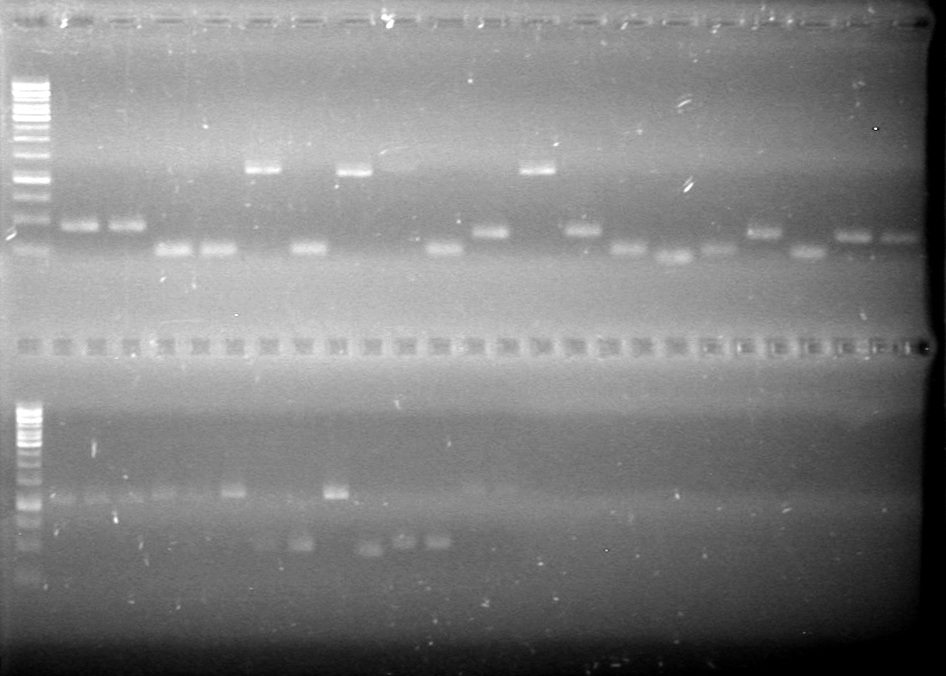
Ran gel at 100V for 45 minutes.<br> | Ran gel at 100V for 45 minutes.<br> | ||
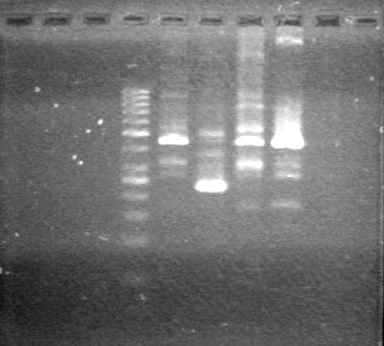
| - | < | + | <b>Results:</b> |
| + | [[image:Lethbridge_100705AVPCR.JPG|200px]]<br> | ||
| - | ==July 5/2010 Evening== | + | ==<font color="white">July 5/2010 Evening== |
| - | <b>Objective:</b>To over-express CFP complete in DH5α<br> | + | <b>Objective:</b> To over-express CFP complete in DH5α<br> |
<b>Method:</b><br> | <b>Method:</b><br> | ||
| Line 109: | Line 268: | ||
2) Went in the shaker at 6:50pm | 2) Went in the shaker at 6:50pm | ||
| - | ==July 6/2010== | + | ==<font color="white">July 6/2010== |
(In lab: JV, AV, HB)<br> | (In lab: JV, AV, HB)<br> | ||
| - | <b>Objective:</b>To continue the over-expression of CFP complete in DH5α<br> | + | <b>Objective:</b> To continue the over-expression of CFP complete in DH5α<br> |
<b>Method:</b><br> | <b>Method:</b><br> | ||
| Line 120: | Line 279: | ||
*After checking the the sequencing it was evident that our promotor is always off. It is turned off by the product of the gene TetR. Which is not part of our construct. <br> | *After checking the the sequencing it was evident that our promotor is always off. It is turned off by the product of the gene TetR. Which is not part of our construct. <br> | ||
| - | table><table border ="3"> | + | <table><table border ="3"> |
| - | <tr><td><b>Time (hours)</b></td>OD (600λ)</tr> | + | <tr><td><b>Time (hours)</b></td><td><b>OD (600λ)</b></td></tr> |
<tr><td>0</td><td>0.071</td></tr> | <tr><td>0</td><td>0.071</td></tr> | ||
<tr><td>1</td><td>0.390</td></tr> | <tr><td>1</td><td>0.390</td></tr> | ||
| Line 130: | Line 289: | ||
</table><br> | </table><br> | ||
| - | <b>Results:</b>Ran samples on a 15% SDS-page. The gel did not show any signs of over-expression.<br> | + | <b>Results:</b> Ran samples on a 15% SDS-page. The gel did not show any signs of over-expression.<br> |
| + | |||
| + | |||
| + | <b>Objective:</b> To determine if maxipreps were successful via restriction by NotI and running on 1% Agarose gel<br> | ||
| + | |||
| + | <b>Method:</b><br> | ||
| + | 1) Restriction:<br> | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>DNA</td><td>0.071</td></tr> | ||
| + | <tr><td>Buffer Orange</td><td>0.390</td></tr> | ||
| + | <tr><td>NotI</td><td>0.606</td></tr> | ||
| + | <tr><td>MilliQ H<sub>2</sub>O</td><td>1.250</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | Incubated for 1 hour at 37<sup>o</sup>C. | ||
| + | Heat killed on heat block at 80<sup>o</sup>C for 20 mins.<br> | ||
| + | |||
| + | 2) 1% Agarose gel<br> | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Lane</b></td><td><b>Sample</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>1</td><td>1 kb Ladder</td><td>0.5 Ladder + 2 Loading dye (6x) + 7.5 MilliQ H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>2</td><td>PET28a restricted</td><td>8 DNA + 2 Loading dye (6x)</td></tr> | ||
| + | <tr><td>3</td><td>PET28a</td><td>8 DNA + 2 Loading dye (6x)</td></tr> | ||
| + | <tr><td>4</td><td>Lumazine restricted</td><td>8 DNA + 2 Loading dye (6x)</td></tr> | ||
| + | <tr><td>5</td><td>Lumazine</td><td>8 DNA + 2 Loading dye (6x)</td></tr> | ||
| + | <tr><td>6</td><td>mms6 restricted</td><td>8 DNA + 2 Loading dye (6x)</td></tr> | ||
| + | <tr><td>7</td><td>mms6</td><td>8 DNA + 2 Loading dye (6x)</td></tr> | ||
| + | <tr><td>8</td><td>xylE restricted</td><td>8 DNA + 2 Loading dye (6x)</td></tr> | ||
| + | <tr><td>9</td><td>xylE</td><td>8 DNA + 2 Loading dye (6x)</td></tr> | ||
| + | <tr><td>10</td><td>Empty</td><td>Empty</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | Ran at 100V for 80 mins.<br> | ||
| + | |||
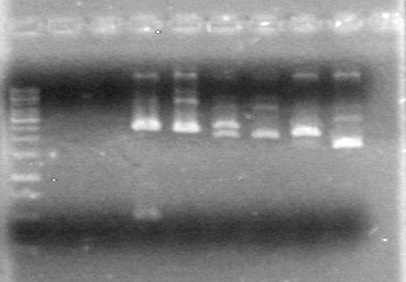
| + | <b>Results:</b> | ||
| + | [[image:Lethbridge_100705AV BW (2).jpg|200px]]<br> | ||
| + | |||
| + | <font color ="Red">More to fill in, but Anthony does not understand the stuff written in the lab book</font><br> | ||
| + | |||
| + | ==<font color="white">July 8/2010== | ||
| + | (In lab: JV, AV, HB, HS)<br> | ||
| + | <b>Objective:</b><br> | ||
| + | |||
| + | <font color ="red"> Anthony does not understand the stuff written in the lab book.</font> | ||
| + | |||
| + | ==<font color="white">July 8/2010 - Evening== | ||
| + | (In lab: KG)<br> | ||
| + | <b>Objective:</b>To transform TetR in pSB1A2 plasmid (BBa_C0040 - 2010 iGEM Distribution Kit Plate Well 4A) and pTetR in pSB1A2 plasmid (BBa_R0040 - 2010 iGEM Distribution Kit Plate Well 6) into DH5α.<br> | ||
| + | |||
| + | <b>Method:</b>Used the Competent Cell Transformation Protocol as well as transformed pUC19 as a positive control.<br> | ||
| + | |||
| + | <b>Results:</b> | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Plate</b></td><td><b>Number of Colonies</b></td></tr> | ||
| + | <tr><td>50 µL TetR</td><td>0</td></tr> | ||
| + | <tr><td>200 µL TetR</td><td>5</td></tr> | ||
| + | <tr><td>50 µL pTetR</td><td>4</td></tr> | ||
| + | <tr><td>200 µL pTetR</td><td>34</td></tr> | ||
| + | <tr><td>50 µL pUC19</td><td>3</td></tr> | ||
| + | <tr><td>200 µL pUC19</td><td>4</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | ==<font color="white">July 9/2010== | ||
| + | (In lab: JV)<br> | ||
| + | <b>Objective:</b>To overexpress pLacI-mRBS-mms6-dT construct.<br> | ||
| + | |||
| + | <b>Method:</b>Used the Overexpression Protocol.<br> | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Time (hours)</b></td><td><b>OD (600λ)</b></td></tr> | ||
| + | <tr><td>0</td><td>0.052</td></tr> | ||
| + | <tr><td>1</td><td>0.111</td></tr> | ||
| + | <tr><td>2</td><td>0.315</td></tr> | ||
| + | <tr><td>2.5</td><td>0.449</td></tr> | ||
| + | <tr><td>3(T<sub>0</sub>)</td><td>0.772</td></tr> | ||
| + | <tr><td>4(T<sub>1</sub>)</td><td>2.22</td></tr> | ||
| + | <tr><td>5(T<sub>2</sub>)</td><td>2.06</td></tr> | ||
| + | <tr><td>6(T<sub>3</sub>)</td><td>2.50</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | Samples were run on a 15% SDS PAGE for 20 minutes at 80V and 1 hour at 200V.<br> | ||
| + | |||
| + | <font color ="red">SDS PAGE picture!!!!!!!!!!!</font> | ||
| + | |||
| + | ==<font color="white">July 10/2010== | ||
| + | (In lab: JV)<br> | ||
| + | <b>Objective:</b>To determine if maxipreps finished on July 9th and 10th have significant concentrations of DNA.<br> | ||
| + | |||
| + | <b>Method:</b>Run 1% Agarose gel<br> | ||
| + | |||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Lane</b></td><td><b>Sample</b></td><td><b>Components (µL)</b></td></tr> | ||
| + | <tr><td>1<td>dT</td><td>2 DNA + 2 loading dye (6x) + 6 MilliQ H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>2<td>mms6 (1)</td><td>2 DNA + 2 loading dye (6x) + 6 MilliQ H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>3<td>mms6 (2)</td><td>2 DNA + 2 loading dye (6x) + 6 MilliQ H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>4<td>pBAD-TetR (1)</td><td>2 DNA + 2 loading dye (6x) + 6 MilliQ H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>5<td>pBAD-TetR (2)</td><td>2 DNA + 2 loading dye (6x) + 6 MilliQ H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>6<td>mRBS</td><td>2 DNA + 2 loading dye (6x) + 6 MilliQ H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>7<td>pLacI</td><td>2 DNA + 2 loading dye (6x) + 6 MilliQ H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>8<td>sRBS</td><td>2 DNA + 2 loading dye (6x) + 6 MilliQ H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>9<td>1 kb Ladder</td><td>0.5 ladder + 2 loading dye (6x) + 7.5 MilliQ H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>10<td>Empty</td><td>Empty</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | Ran at 100V for 40 minutes. Strained in EtBr for 10 minutes.<br> | ||
| + | |||
| + | <b>Results:</b> | ||
| + | [[image:Lethbridge_100710JV BW.JPG|200px]] | ||
| + | |||
| + | ==<font color="white">July 12/2010== | ||
| + | (In lab: AV,HB,JV)<br> | ||
| + | <b>Objective:</b> Maxiprep pLacI (A2) from glycerol stock.<br> | ||
| + | |||
| + | <b>Method:</b> Used Maxiprep Protocol. Cell pellet weighed 1.02g.<br> | ||
| + | |||
| + | <b>Objective:</b> Add dT to the end of mms6, xylE, and lumazine. | ||
| + | |||
| + | <b>Method:</b><br> | ||
| + | 1) Restrict "Part 1" BioBricks: mms6, xylE, and lumazine with EcoRI and SpeI.<br> | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>pDNA</td><td>2</td></tr> | ||
| + | <tr><td>Red Buffer</td><td>2</td></tr> | ||
| + | <tr><td>EcoRI</td><td>0.25</td></tr> | ||
| + | <tr><td>SpeI</td><td>0.25</td></tr> | ||
| + | <tr><td>MilliQ H<sub>2</sub>O</td><td>15.5</td></tr> | ||
| + | </table><br> | ||
| + | 2) Restrict "Part 2" BioBrick: dT with EcoRI and XbaI.<br> | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td><td><b>2.5x Volume (µL)</b></td></tr> | ||
| + | <tr><td>pDNA</td><td>2</td><td>5</td></tr> | ||
| + | <tr><td>Orange Buffer</td><td>2</td><td>5</td></tr> | ||
| + | <tr><td>EcoRI</td><td>0.25</td><td>0.625</td></tr> | ||
| + | <tr><td>XbaI</td><td>0.25</td><td>0.625</td></tr> | ||
| + | <tr><td>MilliQ H<sub>2</sub>O</td><td>15.5</td><td>38.75</td></tr> | ||
| + | </table><br> | ||
| + | 3) 1% Agarose gel (1x TAE) of restricted DNA | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Lane</b></td><td><b>Sample</b></td><td><b>Component (µL)</b></td></tr> | ||
| + | <tr><td>1</td><td>1 kb Ladder</td><td>0.5 ladder + 2 loading dye (6x) + 7.5 MilliQ H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>2</td><td>dT</td><td>8 DNA + 2 loading dye (6x)</td></tr> | ||
| + | <tr><td>3</td><td>dT restricted</td><td>8 DNA + 2 loading dye (6x)</td></tr> | ||
| + | <tr><td>4</td><td>mms6</td><td>8 DNA + 2 loading dye (6x)</td></tr> | ||
| + | <tr><td>5</td><td>mms6 restricted</td><td>8 DNA + 2 loading dye (6x)</td></tr> | ||
| + | <tr><td>6</td><td>xylE</td><td>8 DNA + 2 loading dye (6x)</td></tr> | ||
| + | <tr><td>7</td><td>xylE restricted</td><td>8 DNA + 2 loading dye (6x)</td></tr> | ||
| + | <tr><td>8</td><td>Lumazine</td><td>8 DNA + 2 loading dye (6x)</td></tr> | ||
| + | <tr><td>9</td><td>Lumazine restricted</td><td>8 DNA + 2 loading dye (6x)</td></tr> | ||
| + | <tr><td>10</td><td>Empty</td><td>Empty</td></tr> | ||
| + | </table><br> | ||
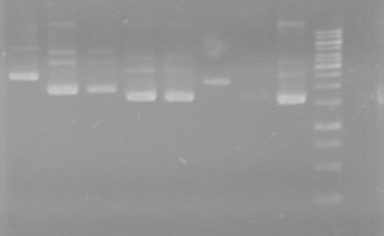
| + | Ran at 100V for 43 minutes. Stained in EtBr for 10 minutes.<br> | ||
| + | <b>Results:</b> | ||
| + | [[image:Lethbridge_100712HB REstriction.JPG|200px]]<br> | ||
| + | |||
| + | 4) Ligate restricted dT to the ends of the "Part 1" Biobricks.<br> | ||
| + | |||
| + | ==<font color="white">July 12/2010 - Evening== | ||
| + | (In lab: KG,TF)<br> | ||
| + | <b>Objective:</b> Continue with addition of dT to the ends of mms6, xylE, and lumazine.<br> | ||
| + | |||
| + | <b>Method:</b><br> | ||
| + | |||
| + | 4) Ligate restricted dT to the ends of the "Part 1" Biobricks.<br> | ||
| + | dT to mms6:<br> | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>T4 DNA Ligase</td><td>0.25</td></tr> | ||
| + | <tr><td>10x T4 Ligation Buffer</td><td>2</td></tr> | ||
| + | <tr><td>dT</td><td>8.3</td></tr> | ||
| + | <tr><td>mms6</td><td>9.2</td></tr> | ||
| + | <tr><td>MilliQ H<sub>2</sub>O</td><td>0.25</td></tr> | ||
| + | </table><br> | ||
| + | dT to xylE:<br> | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>T4 DNA Ligase</td><td>0.25</td></tr> | ||
| + | <tr><td>10x T4 Ligation Buffer</td><td>2</td></tr> | ||
| + | <tr><td>dT</td><td>8.3</td></tr> | ||
| + | <tr><td>xylE</td><td>3.4</td></tr> | ||
| + | <tr><td>MilliQ H<sub>2</sub>O</td><td>6.05</td></tr> | ||
| + | </table><br> | ||
| + | dT to lumazine:<br> | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>T4 DNA Ligase</td><td>0.25</td></tr> | ||
| + | <tr><td>10x T4 Ligation Buffer</td><td>2</td></tr> | ||
| + | <tr><td>dT</td><td>8.3</td></tr> | ||
| + | <tr><td>lumazine</td><td>3.35</td></tr> | ||
| + | <tr><td>MilliQ H<sub>2</sub>O</td><td>6.1</td></tr> | ||
| + | </table><br> | ||
| + | Ligations were incubated at room temperature overnight.<br> | ||
| + | |||
| + | |||
| + | <b>Objective:</b> Prepare mastermix for four PCR reactions for following day.<br> | ||
| + | |||
| + | <b>Method:</b> | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td><td><b>5x Volume (µL)</b></td></tr> | ||
| + | <tr><td>10mM dNTPs</td><td>1</td><td>5</td></tr> | ||
| + | <tr><td>5x Phusion Buffer</td><td>4</td><td>20</td></tr> | ||
| + | <tr><td>Forward Primer (VF2)</td><td>1</td><td>5</td></tr> | ||
| + | <tr><td>Reverse Primer (VR)</td><td>1</td><td>5</td></tr> | ||
| + | <tr><td>MilliQ H<sub>2</sub>O</td><td>10.8</td><td>54</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | ==<font color="white">July 13/2010== | ||
| + | (in lab: JV)<br> | ||
| + | <b>Objective:</b> To determine if ligations of previous day (July 12/2010) were successful.<br> | ||
| + | <b>Method:</b><br> | ||
| + | 1) Use prepared mastermix to run a PCR of ligated mms6-dT, ligated xylE-dT, ligated lumazine-dT, and control-dT<br> | ||
| + | - To each PCR tube, add 17.8&mirco;L of mastermix, 0.2µL of Phusion DNA polymerase, and 2µL of template DNA.<br> | ||
| + | - Ran PCR's for 36 cycles using the iGEM preset.<br> | ||
| + | |||
| + | 2) 2% Agarose gel<br> | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Lane</b></td><td><b>Sample</b></td><td><b>Components (µL)</b></td></tr> | ||
| + | <tr><td>1</td><td>50 bp Ladder</td><td>1 ladder + 2 loading dye (6x) + 7 MilliQ H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>2</td><td>mms6-dT</td><td>8 PCR product + 2 loading dye (6x)</td></tr> | ||
| + | <tr><td>3</td><td>xylE-dT</td><td>8 PCR product + 2 loading dye (6x)</td></tr> | ||
| + | <tr><td>4</td><td>lumazine-dT</td><td>8 PCR product + 2 loading dye (6x)</td></tr> | ||
| + | <tr><td>5</td><td>dT</td><td>8 PCR product + 2 loading dye (6x)</td></tr> | ||
| + | <tr><td>6</td><td>Empty</td><td>Empty</td></tr> | ||
| + | <tr><td>7</td><td>Empty</td><td>Empty</td></tr> | ||
| + | <tr><td>8</td><td>Empty</td><td>Empty</td></tr> | ||
| + | <tr><td>9</td><td>Empty</td><td>Empty</td></tr> | ||
| + | <tr><td>10</td><td>Empty</td><td>Empty</td></tr> | ||
| + | </table><br> | ||
| + | Ran at 100V for __ minutes. Stained in EtBr for 10 minutes.<br> | ||
| + | |||
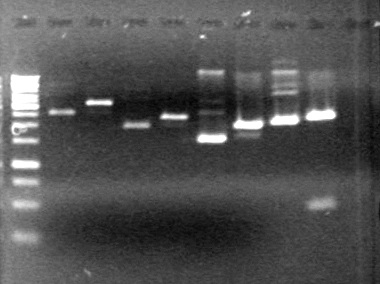
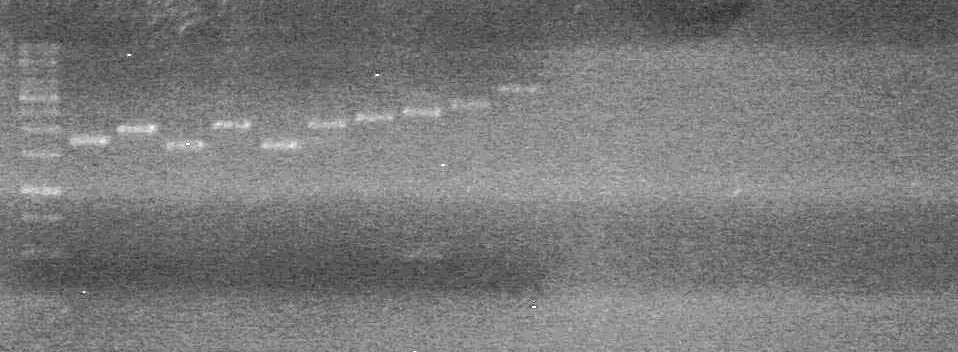
| + | <b>Results:</b> | ||
| + | [[image:Lethbridge_100713JV PCR.JPG|200px]]<br> | ||
| + | |||
| + | ==<font color="white">July 14/2010== | ||
| + | (in lab:J.S, K.G )<br> | ||
| + | <b>Objective:</b> Inoculate culture for maxi prep with placI | ||
| + | |||
| + | <b>Method:</b> | ||
| + | To a 450mL solution of LB media 4.5(µL) of ampicillin was added with glycerol placI aseptically. | ||
| + | ---- | ||
| + | (in lab:J.S, K.G )<br> | ||
| + | <b>Objective:</b> Restriction of dT and mms6 | ||
| + | |||
| + | <b>protocol</b> | ||
| + | |||
| + | 1) | ||
| + | |||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>Buffer&*</td><td>2</td></tr> | ||
| + | <tr><td>10x T4 Milli Q H<sub>2</sub>O</td><td>15.5</td></tr> | ||
| + | <tr><td>pDNA**</td><td>2</td></tr> | ||
| + | <tr><td>Restriction enzymes***</td><td>0.25</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | <b>*</b> Orange buffer was used for dT, and Red buffer was used for mms6 | ||
| + | |||
| + | <b>**</b>pDNA was dT and mms6 | ||
| + | |||
| + | <b>***</b>Restriction enzymes for dT were XbalI and EcoRI, and for mms6 SpeI and EcoRI | ||
| + | |||
| + | 2) | ||
| + | Reaction was incubated at 37<sup>0</sup>C for 1 hour. Start 8:50pm till 9:50pm | ||
| + | After incubation reaction was heat shocked at 80<sup>0</sup>C for 20 minutes | ||
| + | |||
| + | ---- | ||
| + | (in lab:J.S, K.G )<br> | ||
| + | <b>Objective:</b> Ligation of dT to each of mms6, xylE and lumazine. | ||
| + | |||
| + | <b>protocol</b> | ||
| + | |||
| + | 1) | ||
| + | |||
| + | dT to mms6:<br> | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>T4 DNA Ligase</td><td>0.25</td></tr> | ||
| + | <tr><td>10x T4 Ligation Buffer</td><td>2</td></tr> | ||
| + | <tr><td>dT</td><td>8.3</td></tr> | ||
| + | <tr><td>mms6</td><td>9.2</td></tr> | ||
| + | <tr><td>MilliQ H<sub>2</sub>O</td><td>0.25</td></tr> | ||
| + | </table><br> | ||
| + | dT to xylE:<br> | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>T4 DNA Ligase</td><td>0.25</td></tr> | ||
| + | <tr><td>10x T4 Ligation Buffer</td><td>2</td></tr> | ||
| + | <tr><td>dT</td><td>8.3</td></tr> | ||
| + | <tr><td>xylE</td><td>3.4</td></tr> | ||
| + | <tr><td>MilliQ H<sub>2</sub>O</td><td>6.05</td></tr> | ||
| + | </table><br> | ||
| + | dT to lumazine:<br> | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>T4 DNA Ligase</td><td>0.25</td></tr> | ||
| + | <tr><td>10x T4 Ligation Buffer</td><td>2</td></tr> | ||
| + | <tr><td>dT</td><td>8.3</td></tr> | ||
| + | <tr><td>lumazine</td><td>3.35</td></tr> | ||
| + | <tr><td>MilliQ H<sub>2</sub>O</td><td>6.1</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | 2) | ||
| + | Incubated reaction overnight at room temperature<br> | ||
| + | |||
| + | ==<font color="white">July 15/2010== | ||
| + | (in lab: AV)<br> | ||
| + | <b>Objective:</b> Maxiprep pLacI and mms6.<br> | ||
| + | |||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>component</b></td><td><b>pallet weight (g)</b></td></tr> | ||
| + | <tr><td>mms6</td><td>1.02</td></tr> | ||
| + | <tr><td>pLacI</td><td>1.54</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | ==<font color="white">July 15/2010 Evening== | ||
| + | (in lab: AV)<br> | ||
| + | <b>Objective:</b> transform ligations from July 14 and July 12,2010;xylE/dt, mms6/dt lumazine/dt into DH5&alpha. | ||
| + | Also to transform mms6 from July 6 and July 10 into Bl21(DE3) | ||
| + | |||
| + | <b>Protocol:</b> | ||
| + | to transform competent cells see protocol:[https://2010.igem.org/Team:Lethbridge/Notebook/Protocols#Competent_Cell_Transformation] | ||
| + | |||
| + | <b>*</b> cells were incubated on ice for 30 minutes started at 7:00-7:30. | ||
| + | <b>**</b>incubated for 1 hour from 8:00 till 9:00 | ||
| + | |||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>'''Results'''</b></tr> | ||
| + | <tr><td><b>plate</b></td><td><b># of colonies</b></td><td><b>Components (µL)</b></td></tr> | ||
| + | <tr><td>1</td><td>200µL xylE-dt July 12</td><td>0</td></tr> | ||
| + | <tr><td>2</td><td>200µL mms6-dt July 12</td><td>1</td></tr> | ||
| + | <tr><td>3</td><td>200µL lumazine-dt</td><td>1</td></tr> | ||
| + | <tr><td>4</td><td>200µL mms6 maxiprep July 6</td><td>Lawn</td></tr> | ||
| + | <tr><td>5</td><td>200µL positve control puC19 into BL21(DE3)</td><td>Lawn</td></tr> | ||
| + | <tr><td>6</td><td>200µL xylE-dt July 14</td><td>0</td></tr> | ||
| + | <tr><td>7</td><td>200µL mms6-dt July 14</td><td>120</td></tr> | ||
| + | <tr><td>8</td><td>200µL lumazine-dt July14</td><td>0</td></tr> | ||
| + | <tr><td>9</td><td>200µL mms6 maxiprep July 10</td><td>.</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | <b>*</b>because of a shortage of plates not all transformations were plated at 50µL and 200µL | ||
| + | |||
| + | ==<font color="white">July 16,2010== | ||
| + | (in lab: M.C, D.M)<br> | ||
| + | <b>Objective:</b> PCR amplify mms6-dT, xylE-dT, lumazine-dT legations along with dT maxi prep for comparison | ||
| + | |||
| + | <b>Protocol:</b> | ||
| + | |||
| + | Master Mix | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>Milli-Q H<sub>2</sub>O<</td><td>54</td></tr> | ||
| + | <tr><td>Pfu Buffer + MgSO<sub>4</sub></td><td>20</td></tr> | ||
| + | <tr><td>10 mM dMTPs</td><td>5</td></tr> | ||
| + | <tr><td>forward primer</td><td>5</td></tr> | ||
| + | <tr><td>reverse primer</td><td>5</td></tr> | ||
| + | </table><br> | ||
| + | 89 µL TOTAL--> 17.8 into each PCR reaction | ||
| + | |||
| + | + 2 µL ligation | ||
| + | + 2 µL Pfu polymerase | ||
| + | |||
| + | Ran iGem-ligTest in thermocycler | ||
| + | |||
| + | |||
| + | ==<font color="white">July 19, 2010== | ||
| + | (in lab: A.V, H.B)<br> | ||
| + | <b>Objective:</b> Purification pf pLacI and mms6 maxipreps done on July 14 and 16, 2010 using the biobasic protocol for purification of PCR products. | ||
| + | |||
| + | <b>Objective:</b> Add pLacI to sRBS and add dT to xylE and lumazine | ||
| + | |||
| + | <b>Method:</b> | ||
| + | |||
| + | Restrict pLacI, xylE and lumazine with SpeI and EcoRI and restrict dT and sRBS with EcorI and XbaI | ||
| + | |||
| + | 1)Restrictions: | ||
| + | |||
| + | pLacI, xylE, and Lumazine | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>Milli-Q H<sub>2</sub>O</td><td>15.5</td></tr> | ||
| + | <tr><td>Red Buffer<sub>4</sub></td><td>2</td></tr> | ||
| + | <tr><td>SpeI</td><td>0.25</td></tr> | ||
| + | <tr><td>EcoRI</td><td>0.25</td></tr> | ||
| + | <tr><td>pDNA</td><td>2</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | dT | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>Milli-Q H<sub>2</sub>O</td><td>38.75</td></tr> | ||
| + | <tr><td>Orange Buffer<sub>4</sub></td><td>5</td></tr> | ||
| + | <tr><td>XbaI</td><td>0.625</td></tr> | ||
| + | <tr><td>EcoRI</td><td>0.625</td></tr> | ||
| + | <tr><td>pDNA</td><td>5</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | sRBS | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>Milli-Q H<sub>2</sub>O</td><td>15.5</td></tr> | ||
| + | <tr><td>Red Buffer<sub>4</sub></td><td>2</td></tr> | ||
| + | <tr><td>xBal</td><td>0.25</td></tr> | ||
| + | <tr><td>EcoRI</td><td>0.25</td></tr> | ||
| + | <tr><td>pDNA</td><td>2</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | Restriction incubation at 37.5<sup>0</sup>C started at 11:55am. Ended at 12:55pm | ||
| + | Heat killed enzymes at 80<sup>0</sup>C for 20 minutes | ||
| + | |||
| + | 2)Restrictions were run on a 1% agarose gel (1 X TAE) | ||
| + | 1% Agarose gel<br> | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Lane</b></td><td><b>Sample</b></td><td><b>Components (µL)</b></td></tr> | ||
| + | <tr><td>1</td><td>1kB Ladder</td><td>0.5 ladder + 2 loading dye (5x) + 5.5 MilliQ H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>2</td><td>pLacI</td><td>6 pDNA + 2 loading dye (5x)</td></tr> | ||
| + | <tr><td>3</td><td>pLacI restriction digest</td><td>6 pDNA + 2 loading dye (5x)</td></tr> | ||
| + | <tr><td>4</td><td>sRBS</td><td>6 pDNA + 2 loading dye (5x)</td></tr> | ||
| + | <tr><td>5</td><td>sRBS restriction digest</td><td>6 pDNA + 2 loading dye (5x)</td></tr> | ||
| + | <tr><td>6</td><td>xylE</td><td>6 pDNA + 2 loading dye (5x)</td></tr> | ||
| + | <tr><td>7</td><td>xylE restriction digest</td><td>6 pDNA + 2 loading dye (5x)</td></tr> | ||
| + | <tr><td>8</td><td>lumazine</td><td>6 pDNA + 2 loading dye (5x)</td></tr> | ||
| + | <tr><td>9</td><td>lumazine restriction digest</td><td>6 pDNA + 2 loading dye (5x)</td></tr> | ||
| + | <tr><td>10</td><td>dT</td><td>6 pDNA + 2 loading dye (5x)</td></tr> | ||
| + | <tr><td>11</td><td>dT restriction digest</td><td>6 pDNA + 2 loading dye (5x)</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | Gel ran for 70 minutes at 100V and was stained in EtBr for 10 minutes | ||
| + | |||
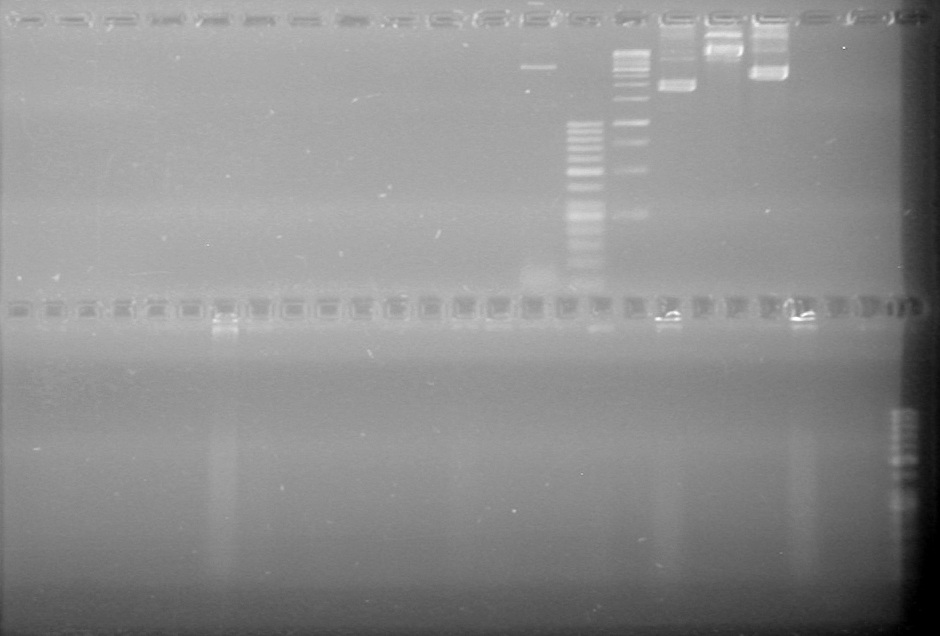
| + | <b>Results:</b> | ||
| + | [[image:Lethbridge_100719HBAVDigest.JPG|200px]]<br> | ||
| + | |||
| + | Results after quantifying restriction digest | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Lane</b></td><td><b>Content</b></td><td><b> Quantity of DNA (ng/µL)</b></td></tr> | ||
| + | <tr><td>1</td><td>1kB Ladder</td><td>12.5</td></tr> | ||
| + | <tr><td>3</td><td>pLacI restriction digest</td><td>5.21</td></tr> | ||
| + | <tr><td>5</td><td>sRBS restriction digest</td><td>3.72</td></tr> | ||
| + | <tr><td>7</td><td>xylErestriction digest</td><td>3.36</td></tr> | ||
| + | <tr><td>9</td><td>Lumazine restriction digest</td><td>2.63</td></tr> | ||
| + | <tr><td>11</td><td>dT restriction digest</td><td>2.98</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | ==<font color="white">July 19, 2010 Evening== | ||
| + | (K.G)<br> | ||
| + | <b>Objective:</b> Ligate together rbs-xylE and dT, lumazine and dT, also pLacI and sRBS.<br> | ||
| + | |||
| + | <b>Method:</b> All ligation mixes had:<br> | ||
| + | *10X T4 Ligation Buffer | ||
| + | *Milli-Q H<sub>2</sub>O to fill to 20µL | ||
| + | *T4 DNA Ligase | ||
| + | *20ng plasmid DNA<br> | ||
| + | |||
| + | Ligations were left over-night at room temperature. <br> | ||
| + | |||
| + | (J.V.)<br> | ||
| + | <b>Objective:</b>Determine the results of the transformations done by K.G. on July 15/2010.<br> | ||
| + | |||
| + | <b>Method:</b> Inoculate 5mL LB media w/Amp and colony. Incubated over night at 37<sup>o</sup>C.<br> | ||
| + | |||
| + | <b>Results:</b> Lumazine Synthase with dT ligated onto it grew.<br> | ||
| + | |||
| + | ==<font color="white">July 20, 2010== | ||
| + | (AV, HB)<br> | ||
| + | <b>Objective:</b>Miniprep lumazine-dt and 4 N-terminus tags and analyze.<br> | ||
| + | |||
| + | <b>Method:</b><br> | ||
| + | |||
| + | *Use [[Team:Lethbridge/Notebook/Protocols|boiling lysis miniprep]] to isolate plasmid DNA. | ||
| + | *PCR amplify BioBrick part to determine if the correct DNA was isolated. | ||
| + | *Ran a 1% Agarose gel to visualize PCR products. | ||
| + | |||
| + | <b><font color="white">Results:</font></b> | ||
| + | Agarose gel did not show any bands.<br> | ||
| + | |||
| + | ==<font color="white">July 20, 2010 Evening== | ||
| + | (AV, HB)<br> | ||
| + | <b>Objective:</b>Transform ligation done on July 19, 2010 in to competent DH5α cells.<br> | ||
| + | * xylE-dT | ||
| + | *lumazine synthase-dT | ||
| + | *pLacI-sRBS | ||
| + | *EYFP and ECFP<br> | ||
| + | |||
| + | <b>Method:</b><br> | ||
| + | *Use [[Team:Lethbridge/Notebook/Protocols|Competent Cell Transformation]] | ||
| + | |||
| + | <b>Results:</b><br> | ||
| + | *xylE-dT no colonies | ||
| + | *pLacI-sRBS | ||
| + | *EYFP | ||
| + | *lumazine-dT | ||
| + | *ECFP | ||
| + | |||
| + | ==<font color="white">July 21, 2010== | ||
| + | (JV)<Br> | ||
| + | <b>Objective:</b> Isolate plasmid DNA from pTet, TetR, pET-28(a). | ||
| + | |||
| + | <b>Method:</b><br> | ||
| + | *Used [[Team:Lethbridge/Notebook/Protocols|Maxiprep]] protocol. | ||
| + | |||
| + | (JV)<br> | ||
| + | |||
| + | <b>Objective:</b> To induce over-expression of pLacI-RBS-Mms6-dt in BL21(DE3) cells.<br> | ||
| + | |||
| + | <b>Method:</b> Used [[Team:Lethbridge/Notebook/Protocols|Overexpression]].<br> | ||
| + | Ran SDS gel for 78 minutes at 200V<br> | ||
| + | |||
| + | ==<font color="white">July 21, 2010 Evening== | ||
| + | (TF, AS)<br> | ||
| + | <b>Objective:</b> Colony PCR to test for insertions of BioBrick construction, and for quality control of parts received from the Registry. Also to prepare DNA to be sent away for sequencing.<br> | ||
| + | <b>Method:</b><br> | ||
| + | -Ran PCR's<br> | ||
| + | -Ran 2% Agarose gel for:.<br> | ||
| + | *K249001<br> | ||
| + | *K249004<br> | ||
| + | *K249005<br> | ||
| + | *K249006<br> | ||
| + | *K249008<br> | ||
| + | *K249014<br> | ||
| + | *K249017<br> | ||
| + | *mms6-dt<br> | ||
| + | *lumazine-dt<br> | ||
| + | *ECFP<br> | ||
| + | *EYFP<br> | ||
| + | *N-term tag<br> | ||
| + | *xylE-dt<br> | ||
| + | *SRBS-pLacI<br> | ||
| + | *dt<br> | ||
| + | *pTet maxiprep<br> | ||
| + | *pET-28(a) maxiprep<br> | ||
| + | *TetR maxiprep<br> | ||
| + | Gel ran at 100V for 60minutes.<br> | ||
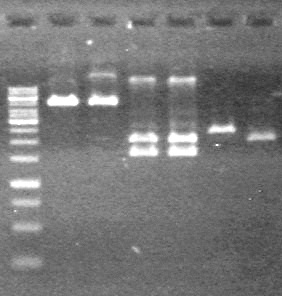
| + | <b>Results:</b> The PCR's did not work. However, the maxipreps show DNA<br> | ||
| + | [[image:Lethbridge_100722 JV BW.jpg|200px]] | ||
| + | |||
| + | ==<font color="white">July 23, 2010== | ||
| + | (JV)<br> | ||
| + | <b>Objective:</b> Insert xylE, Mms6, and lumazine synthase into pET-28(a).<br> | ||
| + | <b>Method:</b> A restriction digest was performed on xylE and Mms6 and ran for 90 minutes at 37 C<br> | ||
| + | <b>Results:</b>Did not see any cut out biobricks.<br> | ||
| + | [[image:Lethbridge_100723MaxiprepRestrictions.jpg|200px]] | ||
| + | |||
| + | ==<font color="white">July 27, 2010== | ||
| + | (in lab: JV, AV, HB)<br> | ||
| + | |||
| + | <b>Objective:</b> To miniprep the overnight cultures of the parts ordered from the Parts Registry and to test the efficiency of the Qiagen Miniprep Kit.<br> | ||
| + | |||
| + | <b>Method:</b> <br> | ||
| + | The following parts were miniprep'd using [[Team:Lethbridge/Notebook/Protocols|Boiling Lysis Plasmid Preparation]]:<br> | ||
| + | *dT (control) | ||
| + | *E0020 (ECFP) | ||
| + | *E0030 (EYFP) | ||
| + | *K249001 | ||
| + | *K249004 | ||
| + | *K249005 | ||
| + | *K249006 | ||
| + | *K249008 | ||
| + | *K249014 | ||
| + | *K249017 | ||
| + | |||
| + | The following parts were miniprep'd using Qiagen Miniprep Kit:<br> | ||
| + | *K249005 | ||
| + | *K249008 | ||
| + | |||
| + | <b>Results:</b> Qiagen Miniprep Kit produced a comparable quantity of DNA to [[Team:Lethbridge/Notebook/Protocols|Boiling Lysis Plasmid Preparation]]. Since the Qiagen Miniprep Kit is faster and easier, all minipreps will be done using the Qiagen Miniprep Kit. | ||
| + | |||
| + | ==<font color="white">July 28, 2010== | ||
| + | (in lab: JV)<br> | ||
| + | |||
| + | <b>Objective:</b> To create a large quantity of pSB1C3 plasmid.<br> | ||
| + | |||
| + | <b>Method:</b> PCR amplified pSB1C3 received from iGEM headquarters. | ||
| + | |||
| + | |||
| + | |||
| + | ==<font color="white">July 29, 2010== | ||
| + | (in lab: HB, AV)<br> | ||
| + | |||
| + | <b>Objective:</b> To PCR amplify the minipreps on July 27, 2010.<br> | ||
| + | |||
| + | <b>Method:</b> PCR amplified the 11 minipreps and dT control.<br> | ||
| + | |||
| + | <b>Results:</b> PCR amplification was successful and 0.2 µL of polymerase will be used per PCR reaction in the future.<br> | ||
| + | |||
| + | <br><br> | ||
| + | <b>Objective:</b> To maxiprep EYFP (E0030), ECFP (E0020), and Lumazine Synthase (K249002).<br> | ||
| + | |||
| + | <b>Method:</b> Used [[Team:Lethbridge/Notebook/Protocols|Maxiprep]]<br> | ||
| + | |||
| + | <table><table border="3"> | ||
| + | <tr><td><b>Cell Pellet</b></td><td><b>Weight (g)</b></td></tr> | ||
| + | <tr><td>ECFP</td><td>2.32</td></tr> | ||
| + | <tr><td>EYFP</td><td>2.00</td></tr> | ||
| + | <tr><td>Lumazine Synthase</td><td>2.66</td></tr></table> | ||
| + | |||
| + | ==<font color="white">July 29, 2010 Evening== | ||
| + | (in lab: KG)<br> | ||
| + | |||
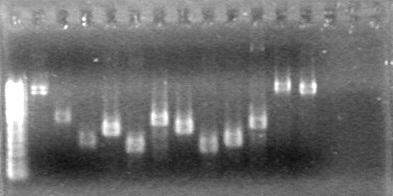
| + | <b>Objective:</b> Run PCR products from the morning on a 2% agarose gel.<br> | ||
| + | |||
| + | <b>Method:</b> Ran 2% agarose gel of the 11 minipreps and dT control and stained in EtBr for 17 minutes.<br> | ||
| + | <b>Results:</b>PCR amplification of last year's parts not consistent with sizes listed on registry.<br> | ||
| + | [[image:Lethbridge_100729 Registry Parts PCR KG.jpg|200px]]<br><br> | ||
| + | <br> | ||
 "
"