Team:Chiba/System 1/Result
From 2010.igem.org
(→Requirement of realizing genetic double click system) |
(→GFP Pulse Generator Result) |
||
| (22 intermediate revisions not shown) | |||
| Line 46: | Line 46: | ||
font: bold 14px Verdana, Arial, Helvetica, sans-serif; | font: bold 14px Verdana, Arial, Helvetica, sans-serif; | ||
color: #000; | color: #000; | ||
| - | margin: | + | margin-top:20px; |
| - | padding: | + | padding-left: 15px; |
} | } | ||
| Line 154: | Line 154: | ||
font: bold 14px Verdana, Arial, Helvetica, sans-serif; | font: bold 14px Verdana, Arial, Helvetica, sans-serif; | ||
color: #000; | color: #000; | ||
| - | margin: | + | margin-top:20px; |
| - | padding: | + | padding-left: 15px; |
} | } | ||
| Line 225: | Line 225: | ||
<ul> | <ul> | ||
<!-- CSS Tabs --> | <!-- CSS Tabs --> | ||
| - | <li><a href="https://2010.igem.org/Team:Chiba/Project"><span> | + | <li><a href="https://2010.igem.org/Team:Chiba/Project"><span>Double Click System</span></a></li> |
| - | <li><a href="https://2010.igem.org/Team:Chiba/System_1"><span> | + | <li><a href="https://2010.igem.org/Team:Chiba/System_1"><span>Version 1</span></a></li> |
| - | <li><a href="https://2010.igem.org/Team:Chiba/System_2"><span> | + | <li><a href="https://2010.igem.org/Team:Chiba/System_2"><span>Version 2</span></a></li> </ul> |
</div> | </div> | ||
</body> | </body> | ||
<!--- 2nd tab End---> | <!--- 2nd tab End---> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
</html> | </html> | ||
| - | |||
<!-- ------------------------------------------------------------------------------- | <!-- ------------------------------------------------------------------------------- | ||
------------------------------------------------------------------------------- | ------------------------------------------------------------------------------- | ||
| Line 343: | Line 238: | ||
------------------------------------------------------------------------------- | ------------------------------------------------------------------------------- | ||
----------------------------------------------------------------------------------- --> | ----------------------------------------------------------------------------------- --> | ||
| + | __NOTOC__ | ||
| + | <br><br> | ||
| + | [[Team:Chiba/System 1|<<Back]] | ||
| + | <br><br> | ||
| + | <font size=5>Version 1 :Evaluating Core Devices</font><br> | ||
| - | <br><br><br> | + | ==<font size="4">Successful operation of this system requires</font>== |
| - | <br><br><br> | + | ===1. Generator of T7 RNA Polymerase pulse=== |
| - | + | In response to both the 1st and the 2nd input, T7 RNAP has to be effective for a moment then get silent. To fulfill this task, | |
| + | *Lux/CI434 hybrid promoter: | ||
| + | **must be activated by LuxR in the presence of AHLs<br> | ||
| + | **must be strictly repressed by CI434<br> | ||
| + | **must NOT be repressed by CI (another repressor components in the circuit)<br> | ||
| + | *CI434 | ||
| + | **should be expressed upon AHL input<br> | ||
| + | **must become effective after a certain time (delayed repression)<br> | ||
| + | **should tightly repress <br> | ||
| + | *T7RNAP | ||
| + | **must be cleared off as soon as possible when its expression is stopped <br> | ||
| - | === | + | ===2. Output module (AND gates of T7RNAP & (NOT CI)) === |
| - | + | *T7/CI promoter should be activated by T7 RNAP | |
| - | + | *T7/CI promoter should be tightly repressed by CI | |
| - | + | *CI should be degraded off after expression shutted off(upon AHL input)<br> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | === | + | ===3. Tuning the timing.=== |
| - | + | *During the T7 RNAP pulse, CI should keep its repression power. ( T7RNAP pulse should be gone before repression by CI start decreasing) | |
| + | *'Invertimer' should create enough time to clear off the repression by CI<br> | ||
| - | + | ==<font size="4"> GFP Pulse Generator Result</font>== | |
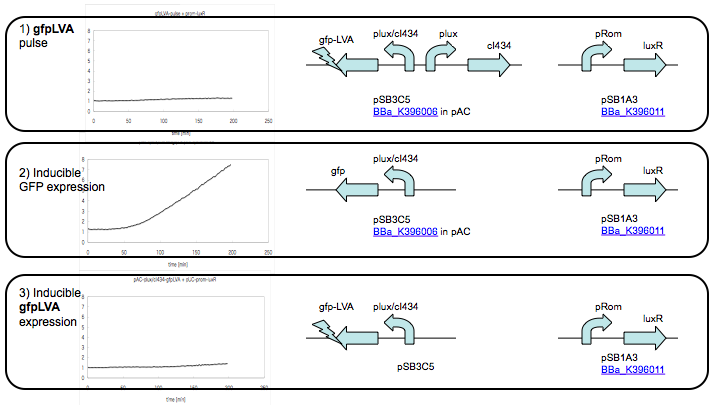
| - | + | *[http://partsregistry.org/wiki/index.php?title=Part:BBa_K396006 BBa_K396006] | |
| + | *[http://partsregistry.org/wiki/index.php?title=Part:BBa_K396011 BBa_K396011] | ||
| + | [[Image:名称未設定.png]] | ||
| - | === | + | ===Protocol=== |
| - | --- | + | E. coli strain DH10B was used for the pulse-generator experiments. |
| - | + | Co-transformed cell were pre-cultured in test tubes for overnight at 37C, 200rpm. | |
| - | + | Dilute the culture 1:100 into 2ml of fresh medium and grow for an additional 5 hours in Supplemented M9 medium . | |
| + | For the liquid experiments, expression was induced at the log phase (OD600 of 0.4) | ||
| + | by the addition of AHL (3OC6HSL)at the appropriate concentration. | ||
| + | One-milliliter samples were taken every 5 min. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | === | + | ===Result=== |
| - | + | By now, there are no obvious pulse observed.The construction we use for measuring GFP is lux/cI434 hybrid promoter following by destabilized GFP. We also check GFP with the same | |
| - | + | degradation tag following by hybrid promoter as a positive control, it neither generate any fluorescent.Maybe because the GFP expression is too low to be investigate because we use | |
| - | + | weak RBS and GFP with LVA.We will continue with investigating GFP pulse. Except that, we | |
| - | + | want to western blot T7 RNA Polymerase pulse and output GFP in plasmid 1 whose transcription | |
| + | factors are T7 RNA Polymerase and cI protein.We hope there will be GFP pulse generated when co-transform plasmid 1 and 2 because of T7 RNA Polymerase amplifier. | ||
Latest revision as of 03:27, 28 October 2010

<<Back
Version 1 :Evaluating Core Devices
Successful operation of this system requires
1. Generator of T7 RNA Polymerase pulse
In response to both the 1st and the 2nd input, T7 RNAP has to be effective for a moment then get silent. To fulfill this task,
- Lux/CI434 hybrid promoter:
- must be activated by LuxR in the presence of AHLs
- must be strictly repressed by CI434
- must NOT be repressed by CI (another repressor components in the circuit)
- must be activated by LuxR in the presence of AHLs
- CI434
- should be expressed upon AHL input
- must become effective after a certain time (delayed repression)
- should tightly repress
- should be expressed upon AHL input
- T7RNAP
- must be cleared off as soon as possible when its expression is stopped
- must be cleared off as soon as possible when its expression is stopped
2. Output module (AND gates of T7RNAP & (NOT CI))
- T7/CI promoter should be activated by T7 RNAP
- T7/CI promoter should be tightly repressed by CI
- CI should be degraded off after expression shutted off(upon AHL input)
3. Tuning the timing.
- During the T7 RNAP pulse, CI should keep its repression power. ( T7RNAP pulse should be gone before repression by CI start decreasing)
- 'Invertimer' should create enough time to clear off the repression by CI
GFP Pulse Generator Result
- [http://partsregistry.org/wiki/index.php?title=Part:BBa_K396006 BBa_K396006]
- [http://partsregistry.org/wiki/index.php?title=Part:BBa_K396011 BBa_K396011]
Protocol
E. coli strain DH10B was used for the pulse-generator experiments. Co-transformed cell were pre-cultured in test tubes for overnight at 37C, 200rpm. Dilute the culture 1:100 into 2ml of fresh medium and grow for an additional 5 hours in Supplemented M9 medium . For the liquid experiments, expression was induced at the log phase (OD600 of 0.4) by the addition of AHL (3OC6HSL)at the appropriate concentration. One-milliliter samples were taken every 5 min.
Result
By now, there are no obvious pulse observed.The construction we use for measuring GFP is lux/cI434 hybrid promoter following by destabilized GFP. We also check GFP with the same degradation tag following by hybrid promoter as a positive control, it neither generate any fluorescent.Maybe because the GFP expression is too low to be investigate because we use weak RBS and GFP with LVA.We will continue with investigating GFP pulse. Except that, we want to western blot T7 RNA Polymerase pulse and output GFP in plasmid 1 whose transcription factors are T7 RNA Polymerase and cI protein.We hope there will be GFP pulse generated when co-transform plasmid 1 and 2 because of T7 RNA Polymerase amplifier.
 "
"