Team:Lethbridge/Notebook/Lab Work/June
From 2010.igem.org
JustinVigar (Talk | contribs) (→June 23/2010) |
Adam.smith4 (Talk | contribs) (→June 28/2010) |
||
| (62 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | <div style="background-color:#000000; color:white"> | |
| + | <html> | ||
| - | + | <br> | |
| - | + | <table border="0" width="100%" style="background-color:#000000"> | |
| - | + | <tr> | |
| - | + | <th> | |
| - | + | <image src="https://static.igem.org/mediawiki/2010/2/29/UofLteamlogo.jpg" width="200px"/> | |
| - | + | ||
| - | + | </th> | |
| - | + | ||
| - | + | ||
| - | + | <th> | |
| - | + | ||
| - | + | ||
| - | + | <image src="https://static.igem.org/mediawiki/2010/9/91/UofLLabWork.JPG" height="300px"/> | |
| - | + | ||
| - | + | ||
| - | + | </th> | |
| - | + | ||
| - | + | ||
| - | + | <th> | |
| - | + | ||
| - | + | ||
| - | + | <image src="https://static.igem.org/mediawiki/2010/2/29/UofLteamlogo.jpg" width="200px"/> | |
| - | + | ||
| - | + | ||
| - | + | </th> | |
| - | + | ||
| - | + | ||
| - | + | </tr> | |
| - | + | ||
| - | + | ||
| - | + | </table> | |
| + | |||
| + | |||
| + | |||
| + | <br> | ||
| + | |||
| + | <align="centre"> | ||
| + | <table border="0" width="100%" style="background-color:#000000"> | ||
| + | |||
| + | <tr> | ||
| + | |||
| + | <th> | ||
| + | |||
| + | <a href="https://2010.igem.org/Team:Lethbridge"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/2/22/UofLHome.jpg" width="80"/> | ||
| + | </a> | ||
| + | |||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Team"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/0/0d/UofLTeam.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Project"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/8/8d/UofLProjectbutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/7/73/UofLNotebookbutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Parts"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/8/84/UofLPartsSubmittedToTheRegistrybutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Modeling"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/e/e1/UofLModelingbutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Ethics"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/2/26/UofLEthicsbutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Safety"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/0/00/UofLSafetybutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Art"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/0/0a/UofLArt.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/News"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/c/c3/UofLNewsButton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | </table> | ||
| + | </body> | ||
| + | </html> | ||
| + | <hr> | ||
| + | |||
| + | <html> | ||
| + | <center> | ||
| + | <font color="white">Feel free to look around our notebook! | ||
| + | </center> | ||
| + | </html> | ||
| + | |||
| + | <html> | ||
| + | <body> | ||
| + | <center> | ||
| + | <table border="0" width="28%" style="background-color:#000000"> | ||
| + | |||
| + | <tr> | ||
| + | <th> | ||
| + | <div class="miniBar"> | ||
| + | <div class="countdown"><object type="application/x-shockwave-flash" data="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=10&date_day=27&date_year=0&un=THE WIKI FREEZE&size=normal&mo=10&da=27&yr=2010" width="300" height="100"><param name="movie" value="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=10&date_day=27&date_year=0&un=THE WIKI FREEZE&size=normal&mo=10&da=27&yr=2010" /><param name="bgcolor" value="#000000" /></object><img src="http://www.oneplusyou.com/q/img/bb_badges/countdown.jpg" alt="" style="display: none;" height="1" width="1" /></div> | ||
| + | <div class="miniContainer"> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/7/73/UofLNotebookbutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Protocols"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/9/91/UofLprotocolsbutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Calendar"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/7/73/UofLcalendar.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th> | ||
| + | <div class="miniBar"> | ||
| + | <div class="countdown"><object type="application/x-shockwave-flash" data="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=11&date_day=05&date_year=0&un=THE IGEM JAMBOREE&size=normal&mo=11&da=05&yr=2010" width="300" height="100"><param name="movie" value="http://www.oneplusyou.com/bb/files/countdown/countdown.swf?co=FFFFFF&bgcolor=000000&date_month=11&date_day=05&date_year=0&un=THE IGEM JAMBOREE&size=normal&mo=11&da=05&yr=2010" /><param name="bgcolor" value="#000000" /></object><img src="http://www.oneplusyou.com/q/img/bb_badges/countdown.jpg" alt="" style="display: none;" height="1" width="1" /></div> | ||
| + | <div class="miniContainer"> | ||
| + | </th> | ||
| + | <tr> | ||
| + | </table> | ||
| + | </center> | ||
| + | </body> | ||
| + | </html> | ||
| + | |||
| + | <hr> | ||
| + | |||
| + | <html> | ||
| + | <center> | ||
| + | <font color="white">Here you can check out the work we have done in the lab, click on a month to take a look! | ||
| + | </center> | ||
| + | </html> | ||
| + | |||
| + | <html> | ||
| + | <body> | ||
| + | <center> | ||
| + | <table border="0" width="50%" style="background-color:#000000"> | ||
| + | |||
| + | <tr> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/April"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/8/8a/UofLapril.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/May"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/7/7b/UofLmaybutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/June"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/8/80/UofLjunebutton.jpg" width="80"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/July"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/5/53/UofLjulybutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/August"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/1/15/UofLaugustbutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/September"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/4/4d/UofLseptemberbutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | <th><a href="https://2010.igem.org/Team:Lethbridge/Notebook/Lab_Work/October"> | ||
| + | <img src="https://static.igem.org/mediawiki/2010/4/4e/UofLoctoberbutton.jpg" width="60"/> | ||
| + | </a> | ||
| + | </th> | ||
| + | |||
| + | |||
| + | |||
| + | <tr> | ||
| + | </table> | ||
| + | </center> | ||
| + | </body> | ||
| + | </html> | ||
| + | <hr> | ||
| - | + | <BLOCKQUOTE> | |
| - | + | ||
| - | + | ||
| - | =June 2010= | + | =<font color="white">June 2010= |
| - | ==June 1/2010== | + | ==<font color="white">June 1/2010== |
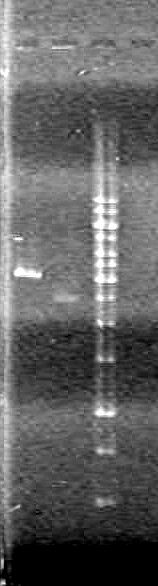
JV quantified the amount of DNA in gels run to date using ImageJ software. Results to be posted in [[Team:Lethbridge/Notebook/Working_Plasmids|working plasmids box]].<br> | JV quantified the amount of DNA in gels run to date using ImageJ software. Results to be posted in [[Team:Lethbridge/Notebook/Working_Plasmids|working plasmids box]].<br> | ||
| Line 68: | Line 218: | ||
*BglII Endonuclease (Bba_K112106)<br> | *BglII Endonuclease (Bba_K112106)<br> | ||
| - | ==June 2/2010== | + | ==<font color="white">June 2/2010== |
(In Lab: JV)<br> | (In Lab: JV)<br> | ||
| Line 141: | Line 291: | ||
Killed enzymes by incubating reactions for 10 minutes at 80<sup>o</sup>C</ul> | Killed enzymes by incubating reactions for 10 minutes at 80<sup>o</sup>C</ul> | ||
| - | ==June 2/2010 - Evening== | + | ==<font color="white">June 2/2010 - Evening== |
<b>Objective:</b> Set up new ligations of pLacI and sRBS-Lum-dT according to Tom Knight's protocol. Previous ligation had very little DNA.<br> | <b>Objective:</b> Set up new ligations of pLacI and sRBS-Lum-dT according to Tom Knight's protocol. Previous ligation had very little DNA.<br> | ||
<b>Relevant Information:</b><br> | <b>Relevant Information:</b><br> | ||
| Line 190: | Line 340: | ||
Incubate for 20 minutes at 80<sup>o</sup>C to heat kill<br> | Incubate for 20 minutes at 80<sup>o</sup>C to heat kill<br> | ||
| - | ==June 3/2010== | + | ==<font color="white">June 3/2010== |
Carried out protocol described in June 2/2010 - Evening<br> | Carried out protocol described in June 2/2010 - Evening<br> | ||
Analyzed results on 1% agarose gel.Load order as follows:<br> | Analyzed results on 1% agarose gel.Load order as follows:<br> | ||
| Line 220: | Line 370: | ||
| - | ==June 3/2010 - Evening== | + | ==<font color="white">June 3/2010 - Evening== |
<b>Objective:</b> Repeat restriction of pSB1T3 and ligate with pLacI and sRBS-Lum-dT. Previous ligations all used up on gel.<br> | <b>Objective:</b> Repeat restriction of pSB1T3 and ligate with pLacI and sRBS-Lum-dT. Previous ligations all used up on gel.<br> | ||
<b>Method:</b><br> | <b>Method:</b><br> | ||
| Line 265: | Line 415: | ||
<b>Continue Ligation on Saturday (See below).</b><br> | <b>Continue Ligation on Saturday (See below).</b><br> | ||
| - | ==June 5/2010 == | + | ==<font color="white">June 5/2010 == |
(In the lab:AS)<br> | (In the lab:AS)<br> | ||
<b>Objective:</b> Ligate restriction products from June 3/2010.<br> | <b>Objective:</b> Ligate restriction products from June 3/2010.<br> | ||
| Line 289: | Line 439: | ||
| - | ==June 6/2010 == | + | ==<font color="white">June 6/2010 == |
(In Lab: JV, HS)<br> | (In Lab: JV, HS)<br> | ||
| Line 321: | Line 471: | ||
None of the plates showed any growth. | None of the plates showed any growth. | ||
| - | ==June 8/2010== | + | ==<font color="white">June 7/2010 == |
| - | (In the lab: JV)<br> | + | (In Lab: JV, HB, TF, AV)<br> |
| + | |||
| + | <b>Objective:</b><br> Purification of DNA to increase ligation and transformation efficiency.<br> | ||
| + | |||
| + | <b>Method:</b><br> BioBasic Protocol for Purification of PCR products.<br> | ||
| + | |||
| + | DNA (from Working Plasmid Box) Purified: | ||
| + | *pBAD-TetR (B10) | ||
| + | *pBAD- TetR (F4) | ||
| + | *pBAD-TetR (F5) | ||
| + | *EYFP (B1) | ||
| + | *pSB-NEYFP (B4) | ||
| + | *pSB-CEYFP (B5) | ||
| + | *NEYFP (E1) | ||
| + | *NEYFP (E2) | ||
| + | *Fusion CEYFP (E3) | ||
| + | *Fusion CEYFP (E4) | ||
| + | *Fusion CEYFP (E5) | ||
| + | *CEYFP (E6) | ||
| + | *CEYFP (E7) | ||
| + | *EYFP (E8) | ||
| + | *EYFP (E9) | ||
| + | *EYFP (E10) | ||
| + | *ECFP (F1) | ||
| + | *ECFP (F2) | ||
| + | *ECFP (F3) | ||
| + | *EYFP (G1) | ||
| + | *pSB-CEYFP (G4) | ||
| + | |||
| + | |||
| + | <b>Objective:</b><br> Restrict and run 1% agarose gel of plasmids J5, J6, and pSB1T3 (June 2/10).<br> | ||
| + | |||
| + | |||
| + | <b>Method:</b> | ||
| + | |||
| + | <b>Restrictions</b> | ||
| + | |||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>Restriction Enzyme (XbaI)</td><td>0.25</td></tr> | ||
| + | <tr><td>Buffer (Tango)</td><td>2</td></tr> | ||
| + | <tr><td>Plasmid DNA</td><td>2</td></tr> | ||
| + | <tr><td>MilliQ H<sub>2</sub>O</td><td>15.75</td></tr> | ||
| + | </table> | ||
| + | |||
| + | Incubated for 1 hour in 37<sup>o</sup>C incubator.<br> | ||
| + | Heat shocked for 10 min at 65<sup>o</sup>C on heating block. | ||
| + | |||
| + | |||
| + | <b>1% Agarose Gel</b> | ||
| + | |||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Lane</b></td><td><b>Sample</b><td><b>Load (µL)</b></td></tr> | ||
| + | <tr><td>1</td><td>1kb ladder</td><td>2 ladder + 2 dye (6X) + 8 MilliQ H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>2</td><td>J5-pLacI-SRBS-Lumazine-dT(1)</td><td>10 DNA + 2 Dye (6X)</td></tr> | ||
| + | <tr><td>3</td><td>J5-pLacI-SRBS-Lumazine-dT(1)restricted</td><td>10 DNA + 2 Dye (6X)</td></tr> | ||
| + | <tr><td>4</td><td>J6-pLacI-SRBS-Lumazine-dT(2)</td><td>10 DNA + 2 Dye (6X)</td></tr> | ||
| + | <tr><td>5</td><td>J5-pLacI-SRBS-Lumazine-dT(2)restricted</td><td>10 DNA + 2 Dye (6X)</td></tr> | ||
| + | <tr><td>6</td><td>pSB1T3</td><td>10 DNA + 2 Dye (6X)</td></tr> | ||
| + | <tr><td>7</td><td>pSB1T3 restricted</td><td>10 DNA + 2 Dye (6X)</td></tr> | ||
| + | <tr><td>8</td><td>Empty</td><td></td></tr> | ||
| + | <tr><td>9</td><td>Empty</td><td></td></tr> | ||
| + | <tr><td>10</td><td>Empty</td><td></td></tr></table> | ||
| + | |||
| + | Ran at 100V for 72 min.<br> | ||
| + | |||
| + | <font color ="Red">IMAGE TO COME!!!!!!</font><br> | ||
| + | |||
| + | ==<font color="white">June 7/2010 - Evening == | ||
| + | (In Lab: AS, KG)<br> | ||
| + | |||
| + | <b>Objective:</b><br> Ligation of:<br> | ||
| + | *pBAD-TetR (F5) + CEYFP (E6) | ||
| + | *pBAD-TetR (F5) + NEYFP (E1) | ||
| + | *pBAD-TetR (F5) + pSB-NEYFP (B4) | ||
| + | *pBAD-TetR (F5) + Fusion-CEYFP (E7) | ||
| + | *pBAD-TetR (F5) + Fusion-CEYFP (E3) | ||
| + | *pBAD-TetR (F5) + CEYFP (E7) | ||
| + | *pBAD-TetR (F5) + NEYFP (E2) | ||
| + | *pBAD-TetR (F5) + pSB-CEYFP (B5) | ||
| + | *pBAD-TetR (F5) + Fusion-CEYFP (E5) | ||
| + | *pBAD-TetR (F5) + pSB-CEYFP (G4) | ||
| + | *pBAD-TetR (F4) + CEYFP (E7) | ||
| + | *pBAD-TetR (F4) + NEYFP (E1) | ||
| + | *pBAD-TetR (F4) + pSB-NEYFP (B4) | ||
| + | *pBAD-TetR (F4) + pSB-CEYFP (B5) | ||
| + | *pBAD-TetR (F4) + CEYFP (E6) | ||
| + | *pBAD-TetR (F4) + Fusion-CEYFP (E4) | ||
| + | *pBAD-TetR (F4) + Fusion-CEYFP (E3) | ||
| + | *pBAD-TetR (F4) + pSB-CEYFP (G4) | ||
| + | *pBAD-TetR (F4) + Fusion-CEYFP (E5) | ||
| + | *pBAD-TetR (F4) + NEYFP (E2) | ||
| + | *pBAD-TetR (B10) + pSB-NEYFP (B4) | ||
| + | *pBAD-TetR (B10) + pSB-CEYFP (G4) | ||
| + | *pBAD-TetR (B10) + NEYFP (E1) | ||
| + | *pBAD-TetR (B10) + CEYFP (E6) | ||
| + | *pBAD-TetR (B10) + CEYFP (E7) | ||
| + | *pBAD-TetR (B10) + Fusion-CEYFP (E4) | ||
| + | *pBAD-TetR (B10) + pSB-CEYFP (B5) | ||
| + | *pBAD-TetR (B10) + Fusion-CEYFP (E5) | ||
| + | *pBAD-TetR (B10) + Fusion-CEYFP (E3) | ||
| + | *pBAD-TetR (B10) + NEYFP (E2) | ||
| + | |||
| + | <b>Method:</b><br> | ||
| + | <font color ="Red">FILL ME IN!!!!!!</font><br> | ||
| + | |||
| + | ==<font color="white">June 8/2010== | ||
| + | (In the lab: JV, AV)<br> | ||
<b>Objective:</b> Follow the overexpression of our pLacI-sRBS-Lum-dT construct.<br> | <b>Objective:</b> Follow the overexpression of our pLacI-sRBS-Lum-dT construct.<br> | ||
<b>Method:</b> <font color ="Red">FILL ME OUT!!!!!!</font><br> | <b>Method:</b> <font color ="Red">FILL ME OUT!!!!!!</font><br> | ||
| Line 435: | Line 692: | ||
Looks like the mms6 DNA was not cut at all. Therefore is doubtful that the ligations will work.<br><br> | Looks like the mms6 DNA was not cut at all. Therefore is doubtful that the ligations will work.<br><br> | ||
| - | ==June 8/2010 - Evening== | + | ==<font color="white">June 8/2010 - Evening== |
<b>Objective:</b> Transform pLacI-sRBS-Lum-dT constructs assembled via three antibiotic assembly on June 3/2010. Also transform mms6-dT constructs assembled today using old insertion method.<br> | <b>Objective:</b> Transform pLacI-sRBS-Lum-dT constructs assembled via three antibiotic assembly on June 3/2010. Also transform mms6-dT constructs assembled today using old insertion method.<br> | ||
<b>Method:</b> Follow [[Team:Lethbridge/Notebook/Protocols|transformation protocol]]. | <b>Method:</b> Follow [[Team:Lethbridge/Notebook/Protocols|transformation protocol]]. | ||
<b>Results:</b> No colonies anywhere<br> | <b>Results:</b> No colonies anywhere<br> | ||
| - | ==June 9/2010== | + | ==<font color="white">June 9/2010== |
(in the lab: TF, JV)<br> | (in the lab: TF, JV)<br> | ||
<b>Objective:</b> Transform mms6-dT ligation reactions from June 8/2010.<br> | <b>Objective:</b> Transform mms6-dT ligation reactions from June 8/2010.<br> | ||
| Line 450: | Line 707: | ||
<b>Results:</b> No transformants on plates.<br><br> | <b>Results:</b> No transformants on plates.<br><br> | ||
| - | ==June 10/2010== | + | ==<font color="white">June 10/2010== |
(In the lab: AV, HB, JV)<br> | (In the lab: AV, HB, JV)<br> | ||
<b>Objective:</b> Repeat ligation of mms6 (B9,D9,D10) and dT (C1).<br> | <b>Objective:</b> Repeat ligation of mms6 (B9,D9,D10) and dT (C1).<br> | ||
| Line 505: | Line 762: | ||
*B0010-B0012: 120 colonies | *B0010-B0012: 120 colonies | ||
| - | ==June 14/2010 Evening== | + | ==<font color="white">June 14/2010 Evening== |
(In the lab: TF, DM, HS)<br> | (In the lab: TF, DM, HS)<br> | ||
<b>Objective:</b> Restriction Digest of RBS-xylE with EcoRI and SpeI<br> | <b>Objective:</b> Restriction Digest of RBS-xylE with EcoRI and SpeI<br> | ||
| Line 529: | Line 786: | ||
*Incubate overnight | *Incubate overnight | ||
| - | ==June 15/2010== | + | ==<font color="white">June 15/2010== |
(In the lab: JV)<br> | (In the lab: JV)<br> | ||
| Line 589: | Line 846: | ||
DNA was then purified using the protocol for purification of PCR products (BioBasic). DNA was eluted with 35µL of elution buffer.<br> | DNA was then purified using the protocol for purification of PCR products (BioBasic). DNA was eluted with 35µL of elution buffer.<br> | ||
| - | ==June 15/2010 Evening== | + | ==<font color="white">June 15/2010 Evening== |
(In the lab: AS)<br> | (In the lab: AS)<br> | ||
| Line 630: | Line 887: | ||
<b>Results: </b>Everything cuts exactly as it should. Continue with ligation and analysis. <font color ="Red">IMAGE TO COME!!!!</font><br> | <b>Results: </b>Everything cuts exactly as it should. Continue with ligation and analysis. <font color ="Red">IMAGE TO COME!!!!</font><br> | ||
| - | ==June 16/2010== | + | ==<font color="white">June 16/2010== |
(in lab: JV) | (in lab: JV) | ||
<b>Objective: </b>To miniprep Adam's overnight cultures of xylE and rbs. Completing this will give us a working stock of this plasmid.<br> | <b>Objective: </b>To miniprep Adam's overnight cultures of xylE and rbs. Completing this will give us a working stock of this plasmid.<br> | ||
| Line 647: | Line 904: | ||
<b>Results:</b> There wasn't any growth after overnight incubation. Possibility of an antibiotic mix-up. | <b>Results:</b> There wasn't any growth after overnight incubation. Possibility of an antibiotic mix-up. | ||
| - | ==June 16/2010 Evening== | + | ==<font color="white">June 16/2010 Evening== |
(in lab: ADS) | (in lab: ADS) | ||
| Line 714: | Line 971: | ||
60µL of each VF2 & VR primers (10µL) -> send 65µL | 60µL of each VF2 & VR primers (10µL) -> send 65µL | ||
| - | ==June 17/2010== | + | ==<font color="white">June 17/2010== |
(in lab: JV, HB) | (in lab: JV, HB) | ||
<b>Objective:</b>Run Agarose gel of overnight samples from June 16/10 to test T4 DNA ligase<br> | <b>Objective:</b>Run Agarose gel of overnight samples from June 16/10 to test T4 DNA ligase<br> | ||
| Line 736: | Line 993: | ||
<font color ="Red">IMAGE TO COME!!!!</font><br> | <font color ="Red">IMAGE TO COME!!!!</font><br> | ||
| - | ==June 17/2010 Evening== | + | ==<font color="white">June 17/2010 Evening== |
(in lab: JS, AS, KG)<br> | (in lab: JS, AS, KG)<br> | ||
| Line 761: | Line 1,018: | ||
*controls (no forward or reverse primer and no DNA)<br> | *controls (no forward or reverse primer and no DNA)<br> | ||
| - | ==June 18/2010== | + | ==<font color="white">June 18/2010== |
(in lab: JV)<br> | (in lab: JV)<br> | ||
| Line 782: | Line 1,039: | ||
<br> | <br> | ||
| - | ==June 21/2010== | + | ==<font color="white">June 21/2010== |
(in lab: JV)<br> | (in lab: JV)<br> | ||
| Line 808: | Line 1,065: | ||
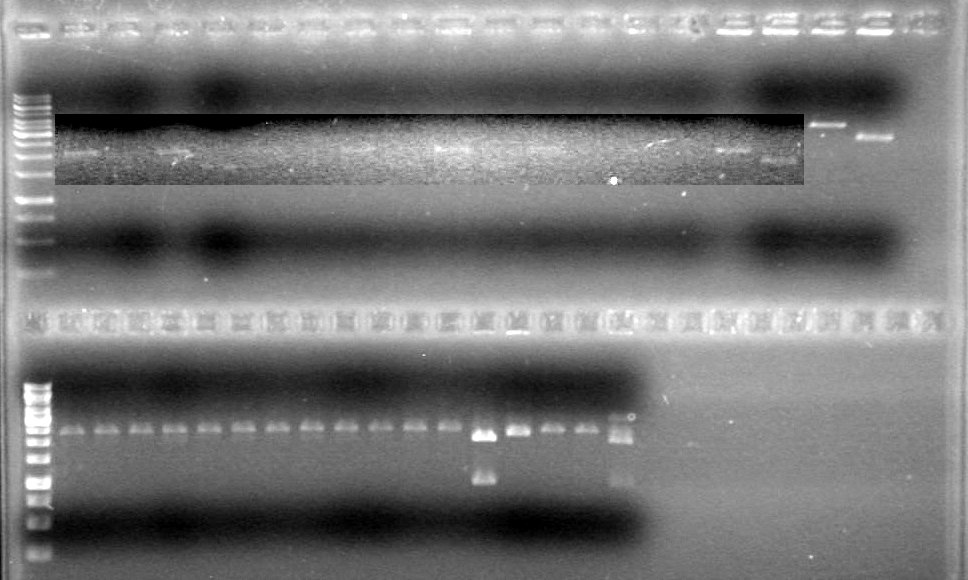
<b>Results:</b>PCR samples were run on a gel (1% 1XTAE) with the PCR samples from June 22/10. This gel was run on June 22/10<br> | <b>Results:</b>PCR samples were run on a gel (1% 1XTAE) with the PCR samples from June 22/10. This gel was run on June 22/10<br> | ||
| - | ==June 22/2010== | + | ==<font color="white">June 22/2010== |
(in lab: JV, HS)<br> | (in lab: JV, HS)<br> | ||
| Line 876: | Line 1,133: | ||
Ran the gel for 40 minutes at 100V.<br> | Ran the gel for 40 minutes at 100V.<br> | ||
| - | ==June 22/2010 Evening== | + | ==<font color="white">June 22/2010 Evening== |
(in lab: KG, AS)<br> | (in lab: KG, AS)<br> | ||
| Line 933: | Line 1,190: | ||
<tr><td>Imitrages polymerase</td><td>0.2</td></tr></table><br> | <tr><td>Imitrages polymerase</td><td>0.2</td></tr></table><br> | ||
| - | ==June 23/2010== | + | ==<font color="white">June 23/2010== |
(in lab: JV, HS)<br> | (in lab: JV, HS)<br> | ||
| Line 991: | Line 1,248: | ||
*all Phusion tubes are <font color ="Blue">blue</font><br> | *all Phusion tubes are <font color ="Blue">blue</font><br> | ||
| - | <b>Results:</b> | + | <b>Results:</b> |
| + | <font color="red">IMAGE!!!</font> | ||
| + | |||
<b>Objective:</b> Adam put in overnight cultures on June 22. I want to extract the plasmid DNA.<br> | <b>Objective:</b> Adam put in overnight cultures on June 22. I want to extract the plasmid DNA.<br> | ||
| Line 999: | Line 1,258: | ||
*All cultures were DH5α in LB w/ Amp. | *All cultures were DH5α in LB w/ Amp. | ||
*Cultures were incubated at 37<sub>o</sub>C. | *Cultures were incubated at 37<sub>o</sub>C. | ||
| - | *Next morning cultures were "minipreped" using [[Team:Lethbridge/Notebook/Protocols|boiling lysis miniprep]] protocol. | + | *Next morning cultures were "minipreped" using [[Team:Lethbridge/Notebook/Protocols|boiling lysis miniprep]] protocol.<br> |
| + | |||
<b>Results:</b> Will view plasmid DNA extracted, on a 1% agarose gel (1X TAE) run at 100V for an hour.<br> | <b>Results:</b> Will view plasmid DNA extracted, on a 1% agarose gel (1X TAE) run at 100V for an hour.<br> | ||
| + | |||
| + | |||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Lane</b></td><td><b>Sample</b></td><td><b>Load (µL)</b></td></tr> | ||
| + | <tr><td>1</td><td>1kb Ladder</td><td>0.5 Ladder + 2 Dye (6X) + 9.5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>2</td><td>CFP complete 2010 box C8</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>3</td><td>Fusion CEYFP 2007 box H5</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>4</td><td>NEYFP 2007 box J6</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>5</td><td>Fusion CEYFP 2007 box J5</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>6</td><td>CFP complete 2010 box A10</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>7</td><td>pSB-NEYFP 2010 box B6 (C1?)</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>8</td><td>Fusion CEYFP 2007 box I5</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>9</td><td>xylE 2007 box B9</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>10</td><td>CEYFP 2007 box G6</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>11</td><td>CFP complete 2010 box A6</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>12</td><td>pSB-CEYFP 2010 box C5</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>13</td><td>pSB-NEYFP 2010 box B6 (C1?)</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>14</td><td>pSB-CEYFP 2010 BOX C3</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>15</td><td>xylE 2007 box C4</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>16</td><td>CEYFP 2007 box H6</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>17</td><td>NEYFP 2007 box J6</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr></table><br> | ||
| + | |||
| + | <font color ="Red">Picture to come!!!!!!!!!</font><br> | ||
| + | |||
| + | ==<font color="white">June 24/2010== | ||
| + | (in lab: JV, HS, AV)<br> | ||
| + | |||
| + | <b>Objective:</b>: To run a PCR of biobrick parts from out working plasmid box.<br> | ||
| + | |||
| + | <b>Method:</b><br> | ||
| + | |||
| + | <table border ="3"> | ||
| + | <tr><td><b>Master Mix</b></td><td><b>Volume/tube (µL)</b></td><td><b>Total Volume (µL)</b></td></tr> | ||
| + | <tr><td>MilliQ H<sub>2</sub>O</td><td>12.8</td><td>448</td></tr> | ||
| + | <tr><td>DNA</td><td>1</td><td>-------</td></tr> | ||
| + | <tr><td>5X Phusion Buffer</td><td>4</td><td>140</td></tr> | ||
| + | <tr><td>10µM dNTP's</td><td>1</td><td>35</td></tr> | ||
| + | <tr><td>Forward Primer</td><td>0.5</td><td>17.5</td></tr> | ||
| + | <tr><td>Reverse Primer</td><td>0.5</td><td>17.5</td></tr> | ||
| + | <tr><td>Polymerase</td><td>0.2</td><td>7</td></tr></table><br> | ||
| + | |||
| + | Ran for 25 cycles on lig25 setting. | ||
| + | |||
| + | <b>Objective:</b>: Restriction Digest, Run on agarose gel, and ligate PCR products from June 23/10.<br> | ||
| + | |||
| + | <b>Method:</b><br> | ||
| + | |||
| + | Six tubes for restriction digest:<br> | ||
| + | <table border ="3"> | ||
| + | <tr><td><b>Part 1</b></td><td><b>Part 2</b></td></tr> | ||
| + | <tr><td>Lane 8 (I2)</td><td>Lane 4 (C1)</td></tr> | ||
| + | <tr><td>Lane 19 (A9)</td><td>Lane 8 (I2)</td></tr> | ||
| + | <tr><td>Lane 19 (A9)</td><td>Lane 8 (I2)</td></tr></table><br> | ||
| + | |||
| + | Restriction Digest Reaction Mixture:<br> | ||
| + | <table border ="3"> | ||
| + | <tr><td><b>Ingredient</b></td><td><b>Volume (µL)</b></td></tr> | ||
| + | <tr><td>Plasmid DNA</td><td>2</td></tr> | ||
| + | <tr><td>Enzyme</td><td>0.5</td></tr> | ||
| + | <tr><td>Buffer</td><td>2</td></tr> | ||
| + | <tr><td>Milli-Q H<sub>2</sub>O</td><td>15.5</td></tr></table><br> | ||
| + | Incubate at 37<sup>o</sup>C for 1 hour. Then heat kill the enzymes at 80<sup>o</sup>C for 20 minutes.<br> | ||
| + | |||
| + | 2% Agarose gel electrophoresis:<br> | ||
| + | |||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Lane</b></td><td><b>Sample</b></td><td><b>Load (µL)</b></td></tr> | ||
| + | <tr><td>1</td><td>50bp Ladder</td><td>1 Ladder + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>2</td><td>Fus-RBS-xylE (I2); Lane 8</td><td>5 PCR sample + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>3</td><td>Fus-RBS-xylE (I2) restricted; Lane 8</td><td>5 PCR sample + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>4</td><td>Phu-dT (C1); Lane 4</td><td>5 PCR sample + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>5</td><td>Phu-dT (C1) restricted; Lane 4</td><td>5 PCR sample + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>6</td><td>Phu-pLacI (A9); Lane 19(1)</td><td>5 PCR sample + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>7</td><td>Phu-pLacI (A9) restricted; Lane 19(1)</td><td>5 PCR sample + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>8</td><td>Phu-pLacI (A9); Lane 19(2)</td><td>5 PCR sample + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>9</td><td>Phu-pLacI (A9) restricted; Lane 19(2)</td><td>5 PCR sample + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>10</td><td>Fus-RBS-xylE (I2); Lane 8(1)</td><td>5 PCR sample + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>11</td><td>Fus-RBS-xylE (I2) restricted; Lane 8(1)</td><td>5 PCR sample + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>12</td><td>Fus-RBS-xylE (I2); Lane 8(2)</td><td>5 PCR sample + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>13</td><td>Fus-RBS-xylE (I2) restricted; Lane 8(2)</td><td>5 PCR sample + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr></table><br> | ||
| + | |||
| + | Ran gel at 100V for 60 min and stained in EtBr for 15 min. | ||
| + | |||
| + | <font color ="Red">Picture to come!!!!!!!!!</font><br> | ||
| + | |||
| + | |||
| + | <b>Objective:</b>: Run 1% agarose gel of PCR samples from June 24/10.<br> | ||
| + | |||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Lane</b></td><td><b>Sample</b></td><td><b>Load (µL)</b></td></tr> | ||
| + | <tr><td>1</td><td>A4-pBAD</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>2</td><td>A4-pBAD</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>3</td><td>A6-SRBS</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>4</td><td>A7-SRBS</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>5</td><td>A8-CFP Complete</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>6</td><td>A10-SRBS</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>7</td><td>B1-EYFP</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>8</td><td>B2-N-term tag</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>9</td><td>B4-pSB-NEYFP</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>10</td><td>B5-pSB-NEYFP</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>11</td><td>B6-CFP</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>12</td><td>B10-pBAD-TetR</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>13</td><td>D3</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>14</td><td>D4-C-term tag</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>15</td><td>D5-C-term tag</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>16</td><td>D6-PLacI</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>17</td><td>E2-NEYFP</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>18</td><td>E6-CEYFP</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>19</td><td>E7-CEYFP</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>20</td><td>1kb ladder</td><td>0.5 ladder + 2 Dye (6X) + 9.5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>21</td><td>E8-EYFP</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>22</td><td>E9-EYFP</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>23</td><td>E10-ECFP</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>24</td><td>F1-ECFP</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>25</td><td>F2-ECFP</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>26</td><td>F3-ECFP</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>27</td><td>F4-pBAD-TetR</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>28</td><td>F5-pBAD-TetR</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>29</td><td>G1-EYFP</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>30</td><td>G4-pSB-CEYFP</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>31</td><td>G6-pBAD(1)</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>32</td><td>G7-pBAD(2)</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>33</td><td>G8-N-term tag</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>33</td><td>G9-Lumazine</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>33</td><td>1kb ladder</td><td>0.5 ladder + 2 Dye (6X) + 9.5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | </table><br> | ||
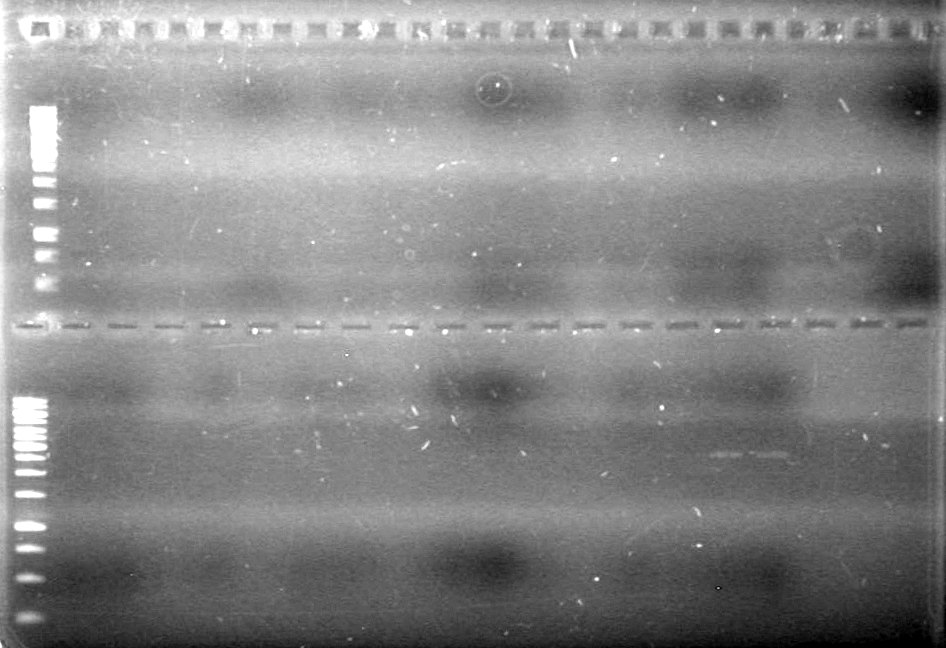
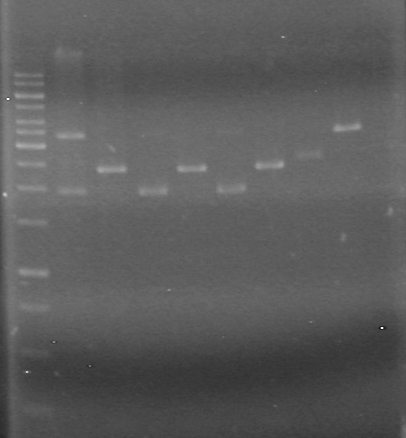
| + | <b>Results:</b> [[image:Lethbridge_100624PCR.JPG|200px]] | ||
| + | |||
| + | ==<font color="white">June 28/2010== | ||
| + | (in lab: JV, HS, AV, HB)<br> | ||
| + | |||
| + | <b>Objective:</b>: To restrict, run 1% agarose gel, and ligate all mms6, lumazine, xylE into pET28(a) for overexpression tests.<br> | ||
| + | |||
| + | <b>Method:</b><br> | ||
| + | 1) Restrict all parts and pET28(a) with NotI<br> | ||
| + | |||
| + | 2) Run on agarose gel (1%)<br> | ||
| + | |||
| + | 3) Ligate parts into pET28(a)<br> | ||
| + | |||
| + | Parts: <br> | ||
| + | :from working plasmid box 2010:<br> | ||
| + | *A3-Lumazine | ||
| + | *B9-Mms6 | ||
| + | *D7-xylE | ||
| + | *D8-xylE | ||
| + | *I10-Mms6 | ||
| + | :from pink tray:<br> | ||
| + | *B9-xylE | ||
| + | *C4-xylE | ||
| + | *G9-Lumazine | ||
| + | :(2X) pET28(a)<br> | ||
| + | |||
| + | Restrictions:<br> | ||
| + | *2µL DNA | ||
| + | *2µL Orange Buffer | ||
| + | *0.25µL Enzyme (NotI) | ||
| + | *15.75µL Milli-Q H<sub>2</sub>O | ||
| + | |||
| + | Protocol:<br> | ||
| + | *1) Incubate for 1 hour at 37<sup>o</sup>C | ||
| + | *2) Heat kill for 20 minutes at 80<sup>o</sup>C (heat block)<br> | ||
| + | |||
| + | Agarose Gel:<br> | ||
| + | |||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Lane</b></td><td><b>Sample</b></td><td><b>Load (µL)</b></td></tr> | ||
| + | <tr><td>1</td><td>1kb ladder</td><td>0.5 ladder + 2 Dye (6X) + 9.5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>2</td><td>Lumazine A3 RD</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>3</td><td>Lumazine A3</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>4</td><td>Mms6 I10 RD</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>5</td><td>Mms6 I10</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>6</td><td>Mms6 B9 RD</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>7</td><td>Mms6 B9</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>8</td><td>xylE D8 RD</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>9</td><td>xylE D8</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>10</td><td>xylE D7 RD</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>11</td><td>xylE D7</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>12</td><td>Lumazine G9 RD (p)</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>13</td><td>Lumazine G9 (p)</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>14</td><td>xylE B9 RD (p)</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>15</td><td>xylE B9 (p)</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>16</td><td>xylE C4 RD (p)</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>17</td><td>xylE C4 (p)</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>18</td><td>pET28(a) RD</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>19</td><td>pET28(a) RD</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr> | ||
| + | <tr><td>20</td><td>pET28(a)</td><td>5 DNA + 2 Dye (6X) + 5 Milli-Q H<sub>2</sub>O</td></tr></table> | ||
| + | |||
| + | Ran gel at 100V for 90 minutes<br> | ||
| + | |||
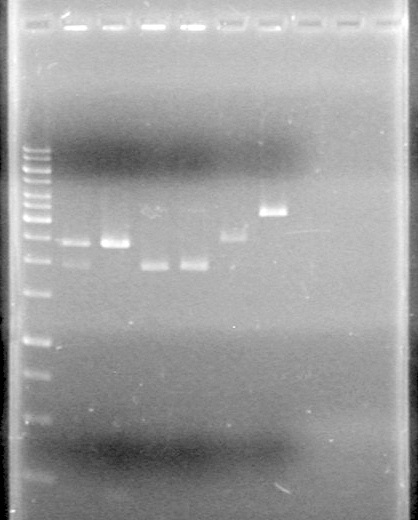
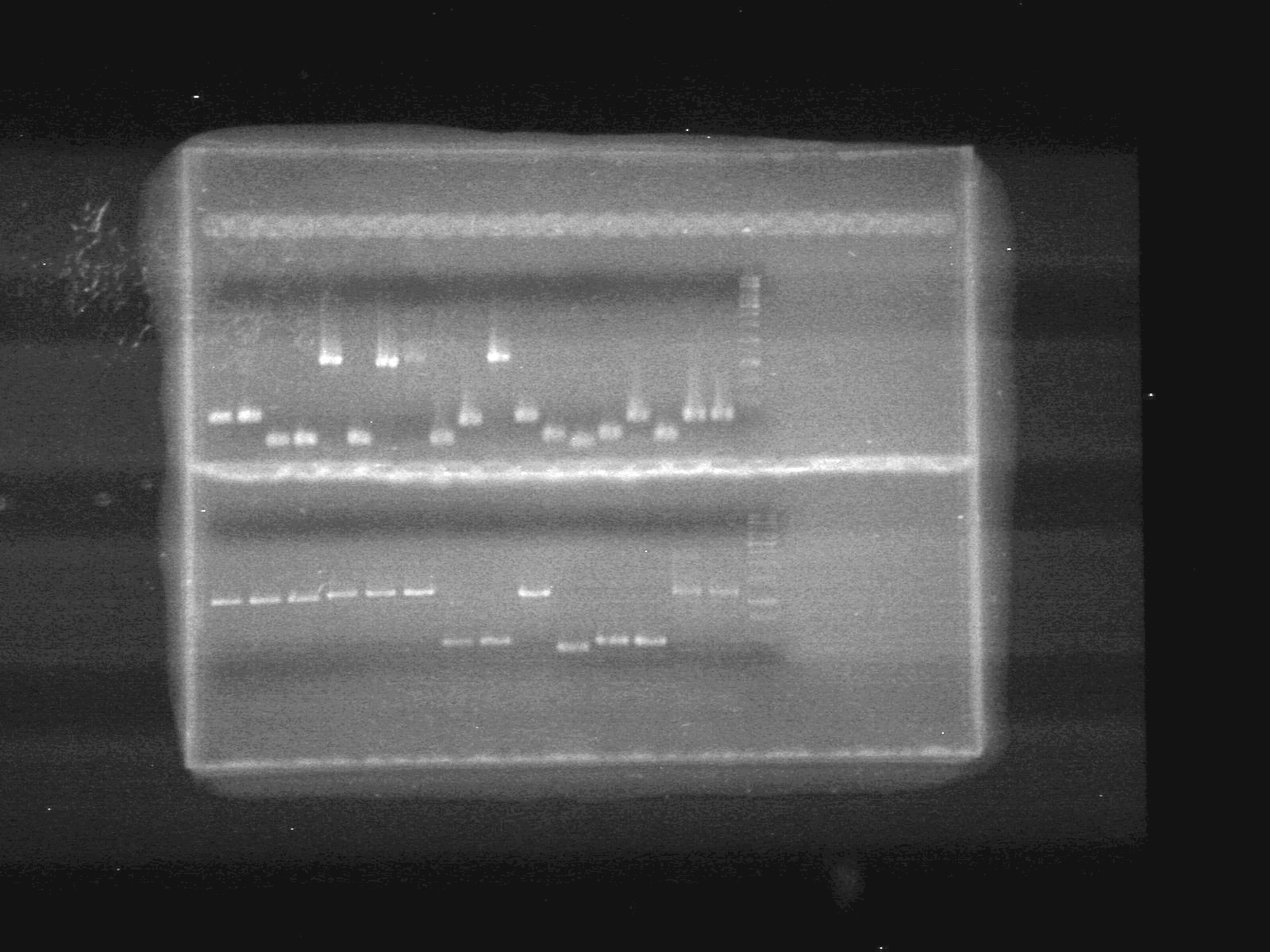
| + | <b>Results:</b>pET28(a) bands are all smeared, and are not useful from inserting parts.<br> | ||
| + | [[image:Lethbridge_100628AV (2).JPG|200px]]<br> | ||
| + | |||
| + | ==<font color="white">June 30/2010== | ||
| + | (in lab: JV)<br> | ||
| + | |||
| + | <b>Objective:</b>: Isolate plasmid DNA from DH5α<br> | ||
| + | |||
| + | <b>Method:</b>Used Kothe Maxiprep protocol.<br> | ||
| + | Cell Pellet Weights:<br> | ||
| + | *xylE = 1.15g | ||
| + | *Mms6 = 1.13g | ||
| + | *pET28(a) = 0.67g | ||
| + | *lumazine synthase = 1.1g<br> | ||
| + | |||
| + | <b>Results:</b><br> | ||
 "
"