Team:Lethbridge/Notebook/Lab Work/September
From 2010.igem.org
(→TF) |
Liszabruder (Talk | contribs) (→AV) |
||
| (19 intermediate revisions not shown) | |||
| Line 279: | Line 279: | ||
</table><br> | </table><br> | ||
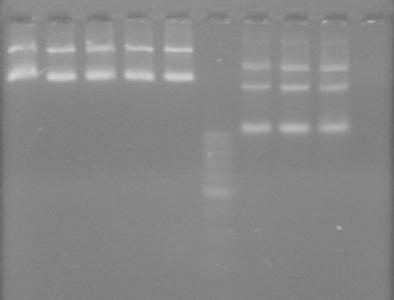
| - | Added 48(µL) to each reaction. | + | Added 48(µL) to each reaction. <br> |
| + | [[image:Lethbridge_100903AssemblyADS.jpg|200px]] | ||
| - | |||
---- | ---- | ||
<b>Objective:</b> Characterize xylE in LB Media vs. M9 Minimal Media.<br> | <b>Objective:</b> Characterize xylE in LB Media vs. M9 Minimal Media.<br> | ||
| Line 336: | Line 336: | ||
(35 cycles) | (35 cycles) | ||
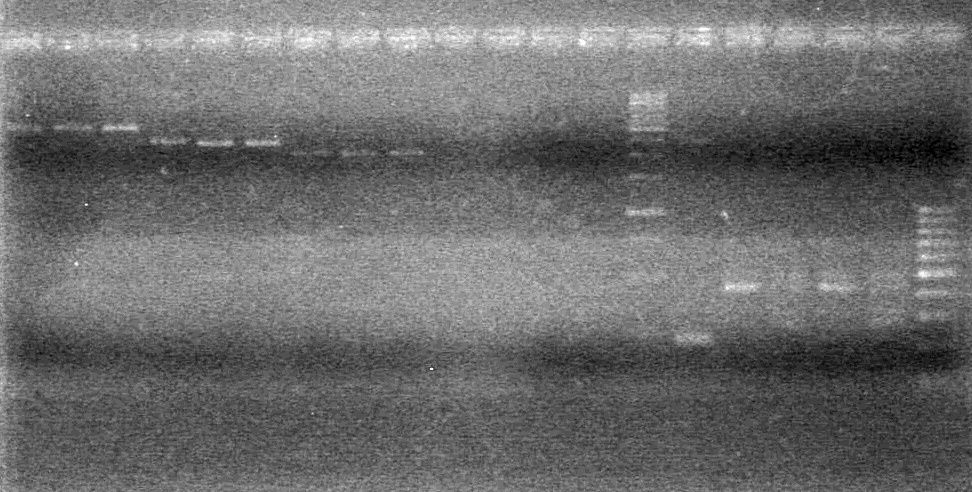
| - | Analyzed PCR on 2% agarose gel which ran at 100 V for 90 minutes. | + | Analyzed PCR on 2% agarose gel which ran at 100 V for 90 minutes.<br> |
| + | [[image:100915PCR AV KG JV.jpg|200px]] | ||
===<font color="white">AS=== | ===<font color="white">AS=== | ||
| Line 428: | Line 429: | ||
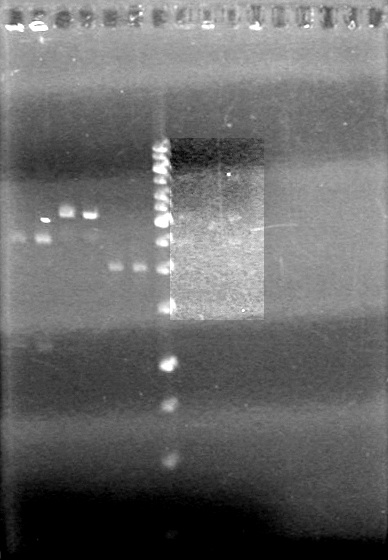
<b>Method:</b> Run 1% TAE agarose gels at 100 V for 90 minutes. <br> | <b>Method:</b> Run 1% TAE agarose gels at 100 V for 90 minutes. <br> | ||
| - | + | [[image:100914ADSAssemblyPrep.jpg|100px]] | |
==<font color="white">September 16, 2010== | ==<font color="white">September 16, 2010== | ||
| Line 436: | Line 437: | ||
<b>Results:</b> Gel of miniprepped RFP against previously prepared RFP showed a band of the right size.<br> | <b>Results:</b> Gel of miniprepped RFP against previously prepared RFP showed a band of the right size.<br> | ||
| - | + | [[image:100916 JV RFP BW.jpg|50px]] | |
==<font color="white">September 17, 2010== | ==<font color="white">September 17, 2010== | ||
| Line 471: | Line 472: | ||
</table> | </table> | ||
| - | + | [[image:100915PCR AV KG JV.jpg|200px]] | |
| - | + | ||
Results: Amplification showing in gel, but fragment amplified was ~1000bp. RFP and dT should be ~800bp. Primers were checked and discovered the YFP/CFP primers were not compatible with the RFP. Concluded the amplification resulted from sloppy annealing of N-term fus prefix to the bio prefix resulting in the full construct being amplified (~1000bp) | Results: Amplification showing in gel, but fragment amplified was ~1000bp. RFP and dT should be ~800bp. Primers were checked and discovered the YFP/CFP primers were not compatible with the RFP. Concluded the amplification resulted from sloppy annealing of N-term fus prefix to the bio prefix resulting in the full construct being amplified (~1000bp) | ||
| Line 556: | Line 556: | ||
---- | ---- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==<font color="white">September 20, 2010== | ==<font color="white">September 20, 2010== | ||
| Line 589: | Line 574: | ||
<tr><td>8</td><td>RFP2</td><td>10</td><td>2</td></tr> | <tr><td>8</td><td>RFP2</td><td>10</td><td>2</td></tr> | ||
<tr><td>9</td><td>RFP3</td><td>10</td><td>2</td></tr></table><br> | <tr><td>9</td><td>RFP3</td><td>10</td><td>2</td></tr></table><br> | ||
| - | <b>Results:</b | + | <b>Results:</b> <br> |
| + | [[image:100920.jpg|100px]] | ||
*mms6 was not amplified | *mms6 was not amplified | ||
*RFP was amplified | *RFP was amplified | ||
| Line 596: | Line 582: | ||
***We believe that the suffix segment of the fusion primer annealed rather than the FP segment. This would cause the promoter (R0040) of J04450 to be amplified, adding ~200bp, giving a final size of >1000bp. | ***We believe that the suffix segment of the fusion primer annealed rather than the FP segment. This would cause the promoter (R0040) of J04450 to be amplified, adding ~200bp, giving a final size of >1000bp. | ||
---------------------------------------------------------------------------------------------------------------------- | ---------------------------------------------------------------------------------------------------------------------- | ||
| - | <b>Objective:</b> Confirm assembly (3 antibiotic) of K118021 and B0015 from Sept. | + | <b>Objective:</b> Confirm assembly (3 antibiotic) of K118021 and B0015 from Sept. 14, 2010 via PCR.<br> |
<b>Method:</b> Set up 50uL reaction mixture with 2uL of ligated DNA. Used VF2 and VR primers.<br> | <b>Method:</b> Set up 50uL reaction mixture with 2uL of ligated DNA. Used VF2 and VR primers.<br> | ||
Used PFU setting on Thermocycler<br> | Used PFU setting on Thermocycler<br> | ||
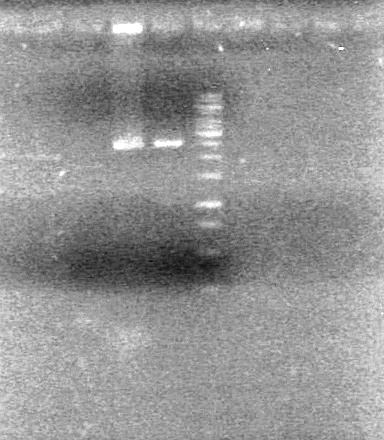
| - | <b>Results:</b | + | <b>Results:</b> <br> |
| + | [[image:100915Assembly.jpg|100px]] | ||
<b>Conclusion:</b> Assembly did not work as intended. | <b>Conclusion:</b> Assembly did not work as intended. | ||
---- | ---- | ||
| + | |||
===<font color="white">HB=== | ===<font color="white">HB=== | ||
| Line 651: | Line 639: | ||
===<font color="white">TF, MC=== | ===<font color="white">TF, MC=== | ||
| - | <b>Objective:</b> Obtain a preparation of B0015 (dT). | + | <b>Objective:</b> Obtain a preparation of B0015 (dT). |
| - | Method:</b> Used [[Team:Lethbridge/Notebook/Protocols|Plasmid DNA Purification by Alkaline Lysis (Large Scale AKA Maxiprep]] protocol.<b> | + | <b>Method:</b> Used [[Team:Lethbridge/Notebook/Protocols|Plasmid DNA Purification by Alkaline Lysis (Large Scale AKA Maxiprep]] protocol. |
| + | ---- | ||
| + | ===<font color="white">AV=== | ||
| + | <b>Objective:</b> PCR amplify the signal sequences synthesized by Mr. Gene (K331007, K331008, K331009, K331012).<br> | ||
| + | |||
| + | Composition of each PCR tube: | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td><b>Volume(µL)</b></td></tr> | ||
| + | <tr><td>10x Pfu Buffer w/ MgSO<sub>4</sub></td><td>2</td></tr> | ||
| + | <tr><td>dNTP (10mM)</td><td>1</td></tr> | ||
| + | <tr><td>VF2</td><td>1</td></tr> | ||
| + | <tr><td>VR</td><td>1</td></tr> | ||
| + | <tr><td>MilliQ H<sub>2</sub>O</td><td>12.8</td></tr> | ||
| + | <tr><td>Template DNA</td><td>2</td></tr> | ||
| + | <tr><td>Pfu DNA Polymerase</td><td>0.2</td></tr> | ||
| + | </table><br> | ||
| + | Used iGEM thermocycler setting: PFU.<br> | ||
==<font color="white">September 21, 2010== | ==<font color="white">September 21, 2010== | ||
| Line 679: | Line 683: | ||
<tr><td>16</td><td>Empty</td><td></td><td></td></tr> | <tr><td>16</td><td>Empty</td><td></td><td></td></tr> | ||
<tr><td>17</td><td>Empty</td><td></td><td></td></tr></table><br> | <tr><td>17</td><td>Empty</td><td></td><td></td></tr></table><br> | ||
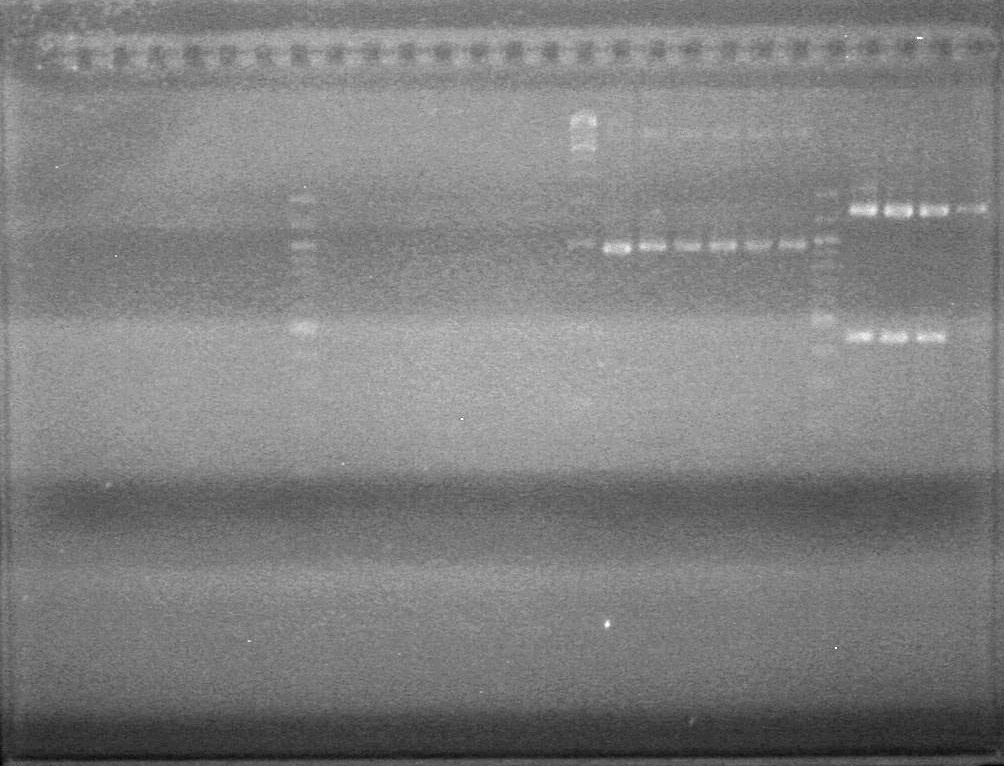
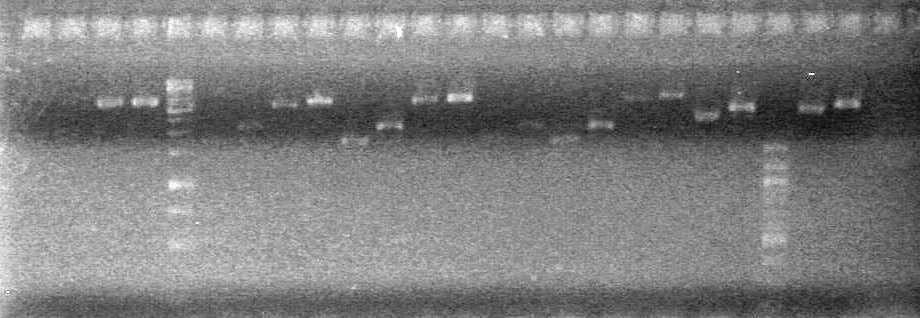
| - | <b>Results:</b | + | <b>Results:</b> <br> |
| + | [[image:100921.jpg|200px]] | ||
*Both KG PCR (Standard-Fusion and Fusion-Standard) amplified | *Both KG PCR (Standard-Fusion and Fusion-Standard) amplified | ||
*ADS PCR Amplified, but no insert present. | *ADS PCR Amplified, but no insert present. | ||
| Line 738: | Line 743: | ||
===<font color="white">TF, MC=== | ===<font color="white">TF, MC=== | ||
| - | <b>Objective:</b> Obtain a preparation of J04450 (RFP).< | + | <b>Objective:</b> Obtain a preparation of J04450 (RFP).<br> |
| - | + | <b>ethod:</b> Used [[Team:Lethbridge/Notebook/Protocols|Plasmid DNA Purification by Alkaline Lysis (Large Scale AKA Maxiprep]] protocol. | |
==<font color="white">September 24, 2010== | ==<font color="white">September 24, 2010== | ||
| Line 815: | Line 820: | ||
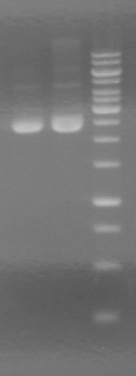
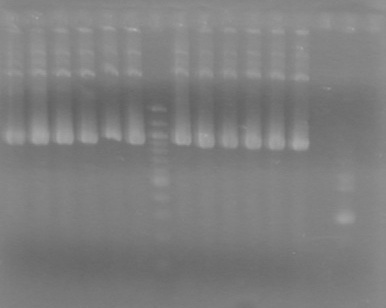
<b>Results:</b> Obtained <font color="red">RESULTS!!!!</font color> positive colonies.<br> | <b>Results:</b> Obtained <font color="red">RESULTS!!!!</font color> positive colonies.<br> | ||
Also analyzed minipreps on 1.5% TAE Agarose Gel<br> | Also analyzed minipreps on 1.5% TAE Agarose Gel<br> | ||
| - | + | [[image:100926Minipreps.jpg|200px]] | |
==<font color="white">September 29, 2010== | ==<font color="white">September 29, 2010== | ||
| Line 828: | Line 833: | ||
Obtained TNTC colonies<br> | Obtained TNTC colonies<br> | ||
Started overnight 5mL cultures for miniprep and insertion into pSB1C3 vector. | Started overnight 5mL cultures for miniprep and insertion into pSB1C3 vector. | ||
| + | |||
| + | ---- | ||
| + | ===<font color="white">AV=== | ||
| + | |||
| + | <b>Objective:</b> PCR the N-term fusion tag onto RFP (E1010) using sloppy annealing of the YFP/CFP fusion primers. <br><br> | ||
| + | |||
| + | Composition of each PCR tube: | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Component</b></td><td>Volume(µL)</td></tr> | ||
| + | <tr><td>10x Pfu Buffer w/ MgSO<sub>4</sub></td><td>2</td></tr> | ||
| + | <tr><td>dNTP (10mM)</td><td>1</td></tr> | ||
| + | <tr><td>N-term Fus Prefix</td><td>1</td></tr> | ||
| + | <tr><td>Biobrick Suffix</td><td>1</td></tr> | ||
| + | <tr><td>MilliQ H<sub>2</sub>O</td><td>12.8</td></tr> | ||
| + | <tr><td>Template DNA (J04450)</td><td>2</td></tr> | ||
| + | <tr><td>Pfu DNA Polymerase</td><td>0.2</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | PCR conditions: | ||
| + | <table><table border ="3"> | ||
| + | <tr><td><b>Step</b></td><td><b>Temperature (<sup>o</sup>C)</b></td><td><b>Time (mins)</b></td><td><b>Number of cycles</b></td></tr> | ||
| + | <tr><td>Initial Denaturation</td><td>95</td><td>2</td><td>1</td></tr> | ||
| + | <tr><td>Denaturation</td><td>95</td><td>0.5</td><td>25</td></tr> | ||
| + | <tr><td>Annealing</td><td>47.6, 50.5, 53.8 (gradient)</td><td>0.5</td><td>25</td></tr> | ||
| + | <tr><td>Extension</td><td>72</td><td>2</td><td>25</td></tr> | ||
| + | <tr><td>Final Extension</td><td>72</td><td>10</td><td>1</td></tr> | ||
| + | </table><br> | ||
| + | |||
| + | Results: Gel has not been run. Have decided to order RFP specific primers for next year.<br><br> | ||
| + | <br> | ||
 "
"