Team:Newcastle/2 July 2010
From 2010.igem.org
(→Protocol) |
(→Competent E. coli Production) |
||
| Line 38: | Line 38: | ||
===Inference=== | ===Inference=== | ||
*''Ecoli'' DH5alpha are kept viable by the colder temperature ready for plasmid extraction after the weekend. | *''Ecoli'' DH5alpha are kept viable by the colder temperature ready for plasmid extraction after the weekend. | ||
| + | |||
==Competent ''E. coli'' Production== | ==Competent ''E. coli'' Production== | ||
| Line 45: | Line 46: | ||
===Materials=== | ===Materials=== | ||
| - | *Liquid culture of ''E. coli'' (DH5alpha strain) | + | *Liquid culture of ''E. coli'' (DH5alpha strain). |
===Protocol=== | ===Protocol=== | ||
| - | *Cause ''E. coli''(DH5alpha strain) to become [[Team:Newcastle/E. coli Competence|competent]] | + | *Cause ''E. coli'' (DH5alpha strain) to become [[Team:Newcastle/E. coli Competence|competent]]. |
===Inference=== | ===Inference=== | ||
| - | *We created a stock of competent E | + | *We created a stock of competent ''E. coli'' (DH5alpha strain) that an be used for future transformations to grow up/replicate plasmids. |
{{Team:Newcastle/footer}} | {{Team:Newcastle/footer}} | ||
Revision as of 10:48, 27 July 2010

| |||||||||||||
| |||||||||||||
Contents |
Urease Test
Aims
The aim of this experiment was to determine if Bacillus subtilis 168 is able to produce urease and degrade urea.
Materials
The experiment was performed on 01.07.10. For the materials used, please see 01.07.10 lab notebook.
Protocol
The experiment was performed on 01.07.10. For the experimental protocol, please see 01.07.10 lab notebook.
Results
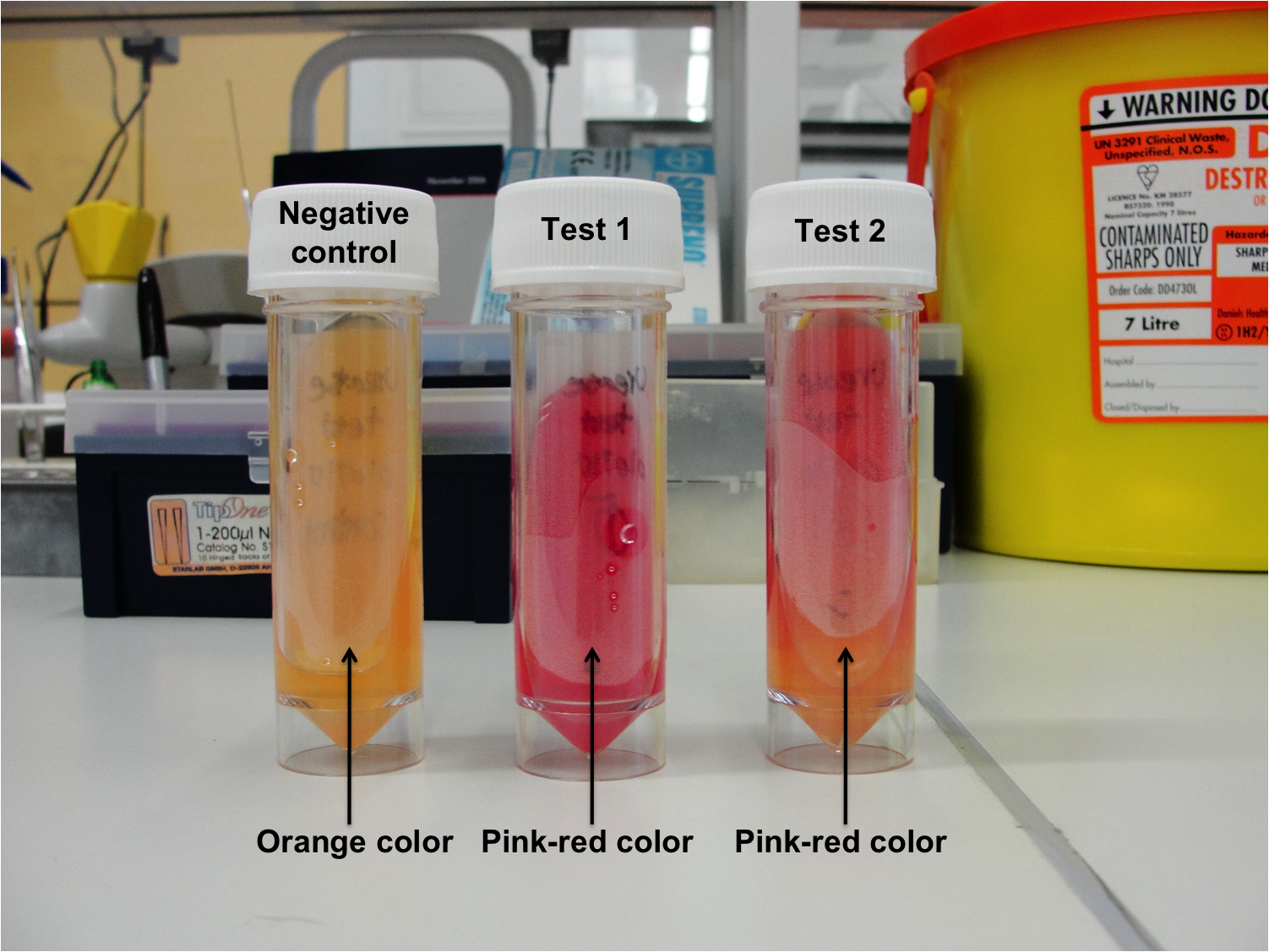
Figure 1: Urease test results
- Negative control - No color change (Orange color)
- Test 1 (Duplicate) - Pink-red color
- Test 2 (Duplicate) - Pink-red color
Discussion
B. subtilis 168 is able to produce urease, therefore, urea which is the substrate and can be found in the agar was degraded, which results in ammonia building. The ammonia makes the media alkaline and therefore the indicator phenol red will change from orange color to pink-red color.
Conclusion
The negative control did not turn pink-red color, therefore indicating that no contamination had occurred.
LacI BioBrick Construction
Aims
- To use PCR to extract lacI (promoter, ribosome-binding site (RBS) & coding sequence (CDS)) from plasmid pMutin4 and ligate into vector pSB1AT3 in front of red fluorescent protein (RFP).
Materials
None
Protocol
- Transformed E. coli DH5alpha are stored in a refrigerator over the weekend.
Inference
- Ecoli DH5alpha are kept viable by the colder temperature ready for plasmid extraction after the weekend.
Competent E. coli Production
Aims
- To make a stock of competent E. coli (DH5alpha strain) ready for transformation.
Materials
- Liquid culture of E. coli (DH5alpha strain).
Protocol
- Cause E. coli (DH5alpha strain) to become competent.
Inference
- We created a stock of competent E. coli (DH5alpha strain) that an be used for future transformations to grow up/replicate plasmids.
 
|
 "
"