Team:Brown/Notebook/July13
From 2010.igem.org
Tuesday, July 13 2010
Double digested Gary’s WillRS ligation, iGEM WillRS ligation, and pNoTat
Reagents used for 20 µl double digest:
- 7.8 µl dH2O
- 2.0 µl 10X NEB Buffer 3
- 8.0 µl DNA
- 1.0 µl NcoI
- 1.0 µl BamHI
- 0.2 µl BSA
20 µl total volume
1 hour in 37°C incubator from 11:00 AM
Inspection of results from last night’s transformation
All kanamycin plates are already filled with a lawn of bacteria, while ampicillin plates are not. Tentatively thinking that the LB/Agar that was prepared was contaminated with a kanamycin-resistant strain.
Transformation of pPTPi
- 4 tubes BL21 (150 µl)
- 2 tubes with 2.5 µl pPTPi
- 2 tubes with 1.0 µl RFP control
13:53 – incubate 30 minutes.
Heat shock 1 minute
Incubate 2 minutes on ice
Add 100 µl LB and plate on 10 µg/mL plates after letting cells recuperate for 30 minutes at 37°C
50 of 10 µg/mL plates: 720 µl of 12.5 mg/mL stock into 900 mL of LB
E.coli need to be grown on 10 µgmL tet! (L. lactis 5 µg/mL tet)
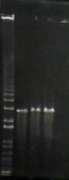
Ran a 1% gel of digest from earlier this morning:
- Lane 1: Ladder (1 kb+)
- Lane 2: pNoTat digest Expect 3 kb
- Lane 3: Gary pGEM digest 3 kb and 800 fragment
- Lane 4: iGEM pGEM digest 3 kb and 800 fragment
Run at 100V. Started at 2:45 PM.
Ran it for a bit too long -- see gel:
1. kb interpretation: one digest worked.
Interpretation: After looking at the ladder (1kb+) we determined we couldn’t see an ~800 bp fragment although pGEM and pNoTat were ~3kb, as expected. This means that our blue-white selection white colonies actually did not have the insert.
To confirm/investigate this possibility, we decided to take Will primers and run a PCR to see if we would get any product (pWill1).
PCR of supposedly ligated pGEM (both iGEM-pGEM and Gary-pGEM) using Will’s primers (labeled RER and REF)
(2X – one for iGEM pGEM, another for Gary pGEM)
- 2 µl fwd primer
- 2 µl rev primer
- 1 µl pGEM (plasmid)
- 25 µl PCR master mix
- 20 µl dH2O
50 µl total volume
PCR program on thermocycler: “IGEM” Start at 7:00 PM.
- Took ~3kb band from gel, pNoTat lane
- Gel extraction protocol on 122 mg of gel
- Stored product as “digested pNoTat, 7/13”
 "
"