Team:Washington/Gram Positive/Test
From 2010.igem.org
(→Test: Enzyme Assay) |
(→Validating CapD_CP Activity) |
||
| Line 36: | Line 36: | ||
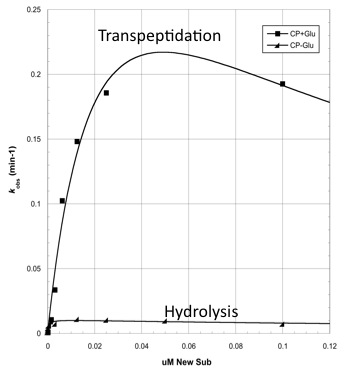
Before we can predict which mutations increase hydrolysis capability, we need to validate that the circularly permuted version of CapD has measurable activity for further assessments. We also hypothesize a threonine residue in the catalytic site of CapD_CP plays an important role in the catalysis reaction and mutating it will eliminate all enzymatic activity. Thus we created two mutants, T2V and T2A, to act as negative controls. The result (figure 1 below) of this assay confirms our hypothesis that CapD_CP has enzymatic activity to the two catalytic knockouts. The relatively flat activity curves of the knockout mutants confirm the hypothesis of the threonine's role in the catalytic site. | Before we can predict which mutations increase hydrolysis capability, we need to validate that the circularly permuted version of CapD has measurable activity for further assessments. We also hypothesize a threonine residue in the catalytic site of CapD_CP plays an important role in the catalysis reaction and mutating it will eliminate all enzymatic activity. Thus we created two mutants, T2V and T2A, to act as negative controls. The result (figure 1 below) of this assay confirms our hypothesis that CapD_CP has enzymatic activity to the two catalytic knockouts. The relatively flat activity curves of the knockout mutants confirm the hypothesis of the threonine's role in the catalytic site. | ||
| - | [[Image:Washington_Confirming_CP_activity_revised3.jpg|thumb|750px|left|Figure 1: Confirming the activity of CapD_CP by comparing it to two CapD_CP knockouts, T2A and T2V.]] | + | [[Image:Washington_Confirming_CP_activity_revised3.jpg|thumb|750px|left|Figure 1: Confirming the activity of CapD_CP by comparing it to two CapD_CP knockouts, T2A and T2V.different graphic is coming]] |
=='''Analyzing CapD_CP'''== | =='''Analyzing CapD_CP'''== | ||
Revision as of 19:08, 29 September 2010
More descriptive titles, verification that your CapD_CP was a monomer ( gel) should go before testing catalytic ability
Contents |
Test: Enzyme Assay

After we have the CapD_CP mutants, we tested our mutants for their catalytic activity using our fluorescence-based enzyme assay scheme. Fluorescence-based enzyme assay measures the rate at which fluorescence in the testing media is released and the amount fluorescence depends on the rate at which fluorophore-quencher linkage is disrupted. Our substrate PDGA contains a linked fluorophore-quencher component. The faster fluorophore-quencher component is cleaved, the higher the amount of fluorescence is released and thus the greater the enzymatic activity observed.
Results: Data Analysis
Validating CapD_CP Activity
Before we can predict which mutations increase hydrolysis capability, we need to validate that the circularly permuted version of CapD has measurable activity for further assessments. We also hypothesize a threonine residue in the catalytic site of CapD_CP plays an important role in the catalysis reaction and mutating it will eliminate all enzymatic activity. Thus we created two mutants, T2V and T2A, to act as negative controls. The result (figure 1 below) of this assay confirms our hypothesis that CapD_CP has enzymatic activity to the two catalytic knockouts. The relatively flat activity curves of the knockout mutants confirm the hypothesis of the threonine's role in the catalytic site.
Analyzing CapD_CP
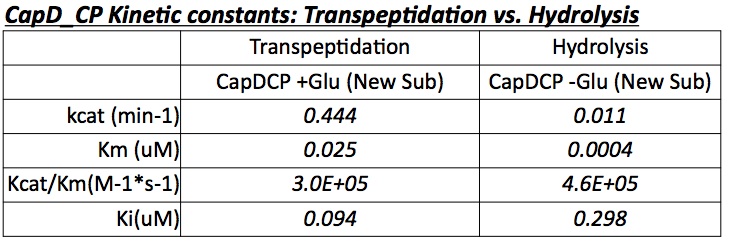
The two abilities of CapD_CP are transpeptidation and hydrolysis. Based on the Kcat and Km (see figure 4) values of the two, we conclude that CapD_CP is a weak binder and efficient catalyst for the transpeptidation reaction. In terms of hydrolysis, it shows strong binding but slow catalysis, thus our mutant designs focus on increasing the catalytic efficiency.
Mutant Designs
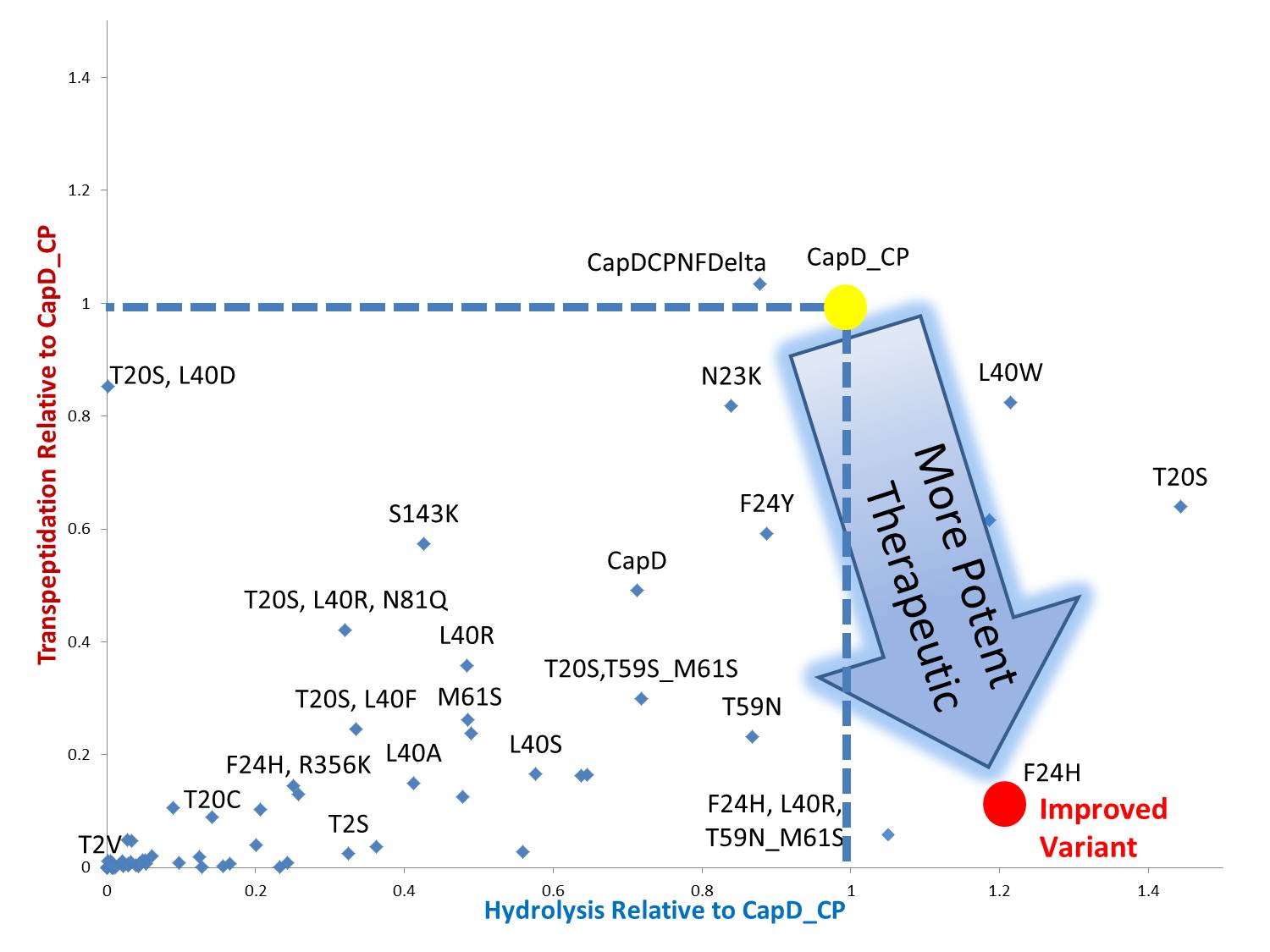
By standardizing the activity slope of each design relative to CapD_CP, a scatterplot easily portrays the qualities of each mutant. Several designs show negative catalytic curves similar to the catalytic knockouts. Some immediately show a negative activity curve meaning decrease in transpeptidation, hydrolysis, or both. T20S is a promising mutant hydrolase design.
 "
"