Team:Washington/Gram Negative/Build
From 2010.igem.org
Using Recombineering to create a constitutive T6SS
The process for using homologous recombination to replace the T6SS native promoters is simple. We begin with a previously characterised fosmid (a large, single-copy plasmid based on the F' plasmid) which contains all the genes in our T6SS. Of particular interest is the fha1-tssA1 intergenic region containing the promoters. We transform this fosmid into recombineering strain E. coli SW102, which contains heat-inducible lambda phage recombinases and lacks the galactose metabolism gene galK.
Using PCR, we create a cassette containing the galK gene flanked by 50 bp of homology to the intergenic region. The lambda recombinases integrate the cassette into the fosmid. The transformants are plated on minimal galactose media to select for the recombinants.
A second cassette is created via PCR, this time containing our new promoters. In this case, we chose to use T7 phage promoters due to their constitutive expression and small size, making them easier to synthesize. The promoters are again flanked with homologous regions, and integrated into the fosmid. This cassette displaces the galK cassette. The transformants are this time plated on 2-deoxy-galactose (DOG), which is a toxic homolog to galactose. Cells which can metabolize galactose (that is, those with a functioning galK gene) are killed, while those without the gene are unharmed.
The final step is to transform the recombinant plasmid into a T7 polymerase expression strain, to allow T6SS constitutive expression.
=Building the Tse2/Tsi2 Toxin/ Antitoxin system
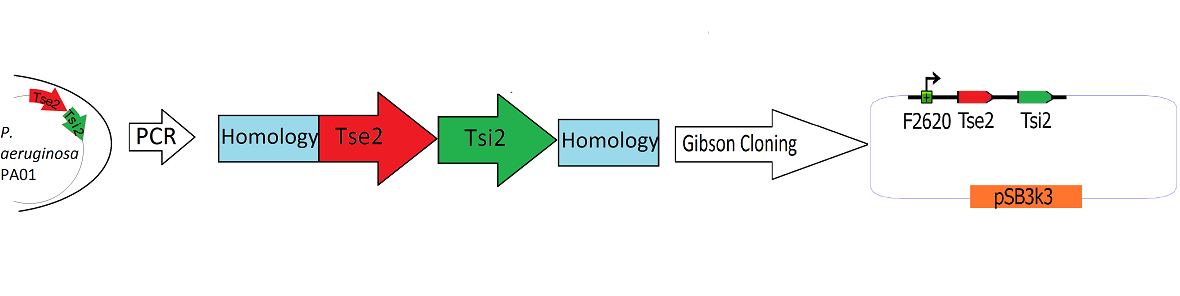
 "
"

