Team:UCL London/Future Vision
From 2010.igem.org
(→Manufacturing) |
(→Manufacturing) |
||
| Line 27: | Line 27: | ||
'''Reliability''' | '''Reliability''' | ||
| - | [[Image:UCL-fao.gif|| | + | [[Image:UCL-fao.gif||300px|right]] |
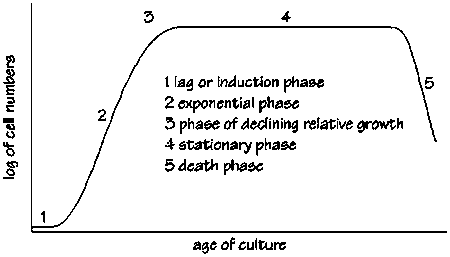
Cells go through different phases before reaching their maximum number. There is no way of telling how long the lag phase would be, or when the oxygen spike would occur. (We experienced a four day lag phase in one of our four fermentations where the cells were not dead, but not growing! This can get frustrating, and can give some ''Steve, the guardian of the fermenters'' horrible ideas like killing the cells and starting again!) | Cells go through different phases before reaching their maximum number. There is no way of telling how long the lag phase would be, or when the oxygen spike would occur. (We experienced a four day lag phase in one of our four fermentations where the cells were not dead, but not growing! This can get frustrating, and can give some ''Steve, the guardian of the fermenters'' horrible ideas like killing the cells and starting again!) | ||
Revision as of 00:18, 11 October 2010
FUTURE VISION
At this stage, our main aim is to show that our circuit does in fact react in the way it does to hypoxia. Pending this achievable target, we have high hopes of our Principle being implemented in a potentially infinite number of ways. We have split them into the following main categories just to give you an idea.
Health & Medicine
Imagine if the bacteria as we know it today could drive protein expression with the lack of oxygen. Synthetic biology through biochemical engineering can create the potential at which Escherichia coli cells- and we all know how harmful to humans can specific strains be- used in a twisted yet beautifully way as an expression system for the production of biopharmaceuticals. The project is based on the engineering modification of the cells and the construction of a self-induced biological circuit that allows an automated protein expression
Manufacturing
Cells need constant monitoring for a "oxygen spike". This peak occurs when cells have reached their maximum growth capacity in the fermenter, and are competing for nutrients in the fermenter. At that point, an operator has to introduce a predetermined volume of IPTG (chemical) into the fermenter to stop the cell growth and channel the energy into transcription of a certain gene. This is how the cells are made to produce pharmaceuticals!
Reliability
Cells go through different phases before reaching their maximum number. There is no way of telling how long the lag phase would be, or when the oxygen spike would occur. (We experienced a four day lag phase in one of our four fermentations where the cells were not dead, but not growing! This can get frustrating, and can give some Steve, the guardian of the fermenters horrible ideas like killing the cells and starting again!)
What if the oxygen spike happens on a weekend, or falls somewhere outside the 8-hour-shift? This is where auto-induction becomes useful. The cells would automatically realise the oxygen spike and change from growth to manufacturing automatically!
Cost
Not only does auto-induction save money on IPTG, it can eliminate the need of IPTG storage saving massive amounts of money on refrigeration in a sterile environment(IPTG must be stored at -20 degrees). Also, there is no more risks of contamination of the IPTG, or of the batch when introducing the IPTG! That means that less batches are wasted, and there is therefore a lower chance of batch failure per year. So with more batches produced per annum, the cost of the drug would decrease giving more people access to it!
Environmental
As mentioned above, IPTG needs to be stored at -20 degrees. Not using IPTG would eliminate the need for refrigerators, making the pharmaceutical industry a little greener.
Online/Real time monitoring
The current method of monitoring the dissolved oxygen in a fermenter is placing several dissolved oxygen probes in different locations of the fermenter. Using the green dye gene, we can reduce the number of probes needed, and depend on a more "live" visual technique!


 "
"





 Twitter
Twitter Facebook
Facebook UCL
UCL Flickr
Flickr YouTube
YouTube