Team:UCL London/Fermenter Mechanics
From 2010.igem.org
| Line 24: | Line 24: | ||
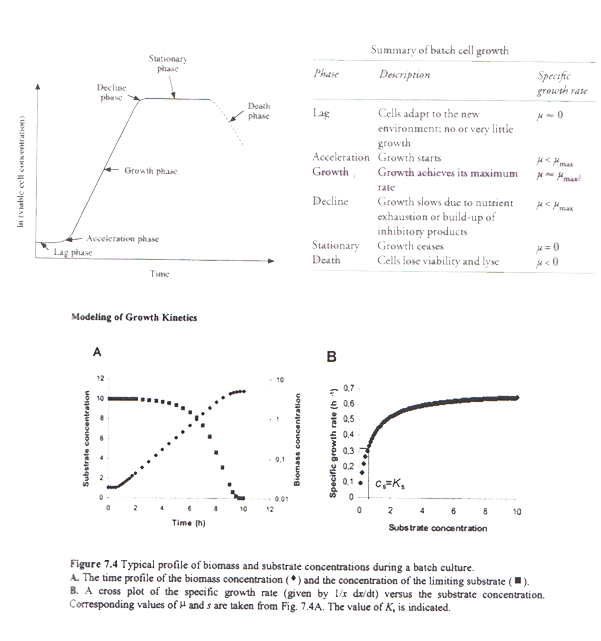
The above graphs, show the modeling of the growth kinetics and the typical profiles of biomass and substrate concentration during a batch process. Reproduced from Nielsen et al (2002), pp:247 | The above graphs, show the modeling of the growth kinetics and the typical profiles of biomass and substrate concentration during a batch process. Reproduced from Nielsen et al (2002), pp:247 | ||
| + | |||
| + | |||
| + | |||
| + | ===Operation Cycle=== | ||
| + | |||
| + | The operation cycle for the fermenter involves start up, where process water is fed from the process water port and media is fed from the feed port into the vessel. The level of the liquid is measured using the level indicator probe. Once the required level is met then the ports close. Air is pumped through the air inlet to the sparger inside the fermenter. | ||
| + | |||
| + | The temperature is brought and maintained to the optimum temperature. There are two probes in the fermenter which are at the greatest range i.e. opposite sides of the fermenter as shown in the drawing. This is to ensure the distribution of heat is even and the average of both sensors should read 37°C. | ||
| + | The pH is maintained at a desired value by the addition of acid and alkali. Two probes for measuring the pH are placed inside the fermenter at opposite ends to ensure there is good mixing as this would be indicated if there was little change between the two probes. | ||
| + | |||
| + | Once the optimum conditions have been achieved the innocculum is fed through the innocculum port. Antifoam from the antifoam port is added to avoid foam formation which reduces the efficiency of the process. The exhaust gases are released from the fermenter whilst in operation and the pressure is maintained. The cells are grown for a certain period of time and the process is regulated using a series of automatic control loops. | ||
| + | |||
| + | Finally there is shut down, where the fermentation batch ends and in this step all the broth is collected into the harvest port. | ||
| + | |||
| + | After each operation cycle the fermenter will have to be cleaned and sterilized in place. CIP will be performed using acid and base detergents which will be pumped through the cleaning solution ports into the 3 spray balls. SIP requires that the fermenter and all pipes are sterilized with steam at 121°C. The steam will come from the acid, base and antifoam ports. | ||
| + | Regular and annual maintenance checks will have to be performed to ensure everything is running correctly and as efficiently as possible. | ||
| + | |||
| + | |||
| + | ===Maintenance=== | ||
| + | |||
| + | Typically, 3-5% of fermentations in an industrial plant are lost due to failure of sterilisation procedures. However in antibiotic fermentations like in this project, fewer than 2% of production scale antibiotic fermentations are lost through contamination by microorganisms or phage. (Doran, 1999) | ||
| + | |||
| + | Industrial fermenters are designed for in situ steam sterilisation under pressure. For effective sterilisation, all air in the vessel and pipe connections must be displaced by steam. After sterilisation, all nutrient medium and air entering the fermenter must be sterile. | ||
| + | |||
| + | Maintenance is required to ensure the fermentation process is achieving its goals, to identify any problems that may occur and ultimately keep the process working as efficiently as possible to save on costs. Maintaining the fermenter can significantly reduce the overall operating cost as well as minimise the risk of any hazards. For maintenance, the top of the fermenter can be removed to allow access of maintenance personnel for any repairs that may be required. For large vessels a domed construction at the top to allow access to personnel is less expensive and so this has been chosen in the design (Doran, 1999). | ||
| + | |||
| + | Once the design has been built and approved by British Standards 5500, the fermenter can then start to operate (Sinott, 1999). Maintenance is crucial and the fermenter must be monitored at all times. Most measurements can be made on-line through probes in the vessel which are sensors that detect changes in many variables such as temperature and pH. However, whilst monitoring some values there may always be a delay. For example, in a typical fermentation, the time scale for change in pH and dissolved-oxygen tension is several minutes to make sure they are all correctly calibrated and functioning properly and as efficiently as possible. This is to avoid any problems that may be caused because of a false reading. For example if the pH probe is not working properly and the pH is either too high or too low, then the cells would die and also the product may denature. Also possible blockages in pipes or broken valves must be checked regularly. Oxygen supply through bottom sparging should also be checked regularly, as cells die without it and that would mean a significant loss in time and money if the fermenter has been running for several days. | ||
| + | |||
| + | The fermenter requires annual shutdown to check for possible corrosion on the internal surface and also on the impellers. Corrosion may reduce the speed and efficiency of them impellers and the shaft too. This needs to be maintained at a high standard to ensure good mixing in the fermenter for growth of cells and product. Baffles also need to be checked for to see the level of corrosion. Also there may be possible fouling in the jackets and general piping problems, such as blockages and leakages, as well as overall contamination in the system. As previously mentioned in the design a 2mm allowance for corrosion had been added on the thickness of all equipment. The annual check would see how much all the equipment has been eroded and identify any problems to resolve them. | ||
| + | |||
| + | The aim of the Mechanical Drawing is to provide a detailed analysis of the construction and operation of the fermenter that is a technical way of presenting this piece of equipment. In this section the dimensions and materials of construction of a 1L fermenter were estimated. The fermenter is used for the growth of Escherichia coli cell culture for the production of therapeutic fragmented antibodies. | ||
| + | |||
| + | For the design project purpose several assumptions had to be made. The first one was the model taken for the scale- up process. This was the original 1L fermenter used for the pilot scale of the E.coli cells. | ||
| + | Assuming that a 1000L fermenter operating with 75% space efficiency (0.75) and 1 marine impeller (di=dv/2). The vessel aspect ratio is 2.5 that is Ah/d= 2.5 and the fermenter is aerated at 0.75 vvm (that is 0.75 volumes of air per volume of liquid per minute). | ||
| + | The gassed power requirement per m3 is Pg/VL= 1860 W/m3 as it was found from the model used for the scale- up. Usually the industrial motors are used to deliver 1-3 W/L (Lye et Baganz, 2007). Therefore the 1.86 W/L was chosen on the assumption that due to engineering cell modifications, the E.coli cells are not so robust and thus extremely high power inputs should be avoided. For this purpose instead of using a marine propeller a lighter choice was preferred since the Chinese Ovary cells are susceptible to mechanical damage (Farid, 2006) due to their large size and lack of rigid cell wall. Therefore the power input value must be kept to a minimum. A Rushton turbine four blade impeller is used as the axial flow type of impeller ensuring medium speed and ensuring medium growth rate but also good mixing. | ||
| + | |||
| + | |||
| + | |||
| + | ===Calculations for determination of the fermenter’s properties=== | ||
| + | |||
| + | The operating pressure of the vessel was assumed to be 3.5 bar and the design pressure 3.85 bar. The operating temperature for mammalian cells is 37°C (Lydersen et al, 1994) but the design temperature is 4- 121°C allowing the introduction of steam to clean the vessel. | ||
| + | |||
| + | Since the mammalian cells are very sensitive, an elevation in temperature e.g. 40°C can cause cell damage and death. Hence temperature probes allowing 〖_-^+〗0.5°C from the temperature set point as well as pH probe to maintain the appropriate range was considered. | ||
| + | |||
| + | |||
| + | [[Image:ucl-sdads.png|center|500px]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
{{:Team:UCL_London/templates/v2/footerFullWidth}} | {{:Team:UCL_London/templates/v2/footerFullWidth}} | ||
Revision as of 22:02, 22 October 2010
Fermentation set-up
Mechanical Design of 1000L Fermenter
This report was conducted to estimate the dimensions and the materials needed for the construction of a 1000L fermenter for the production of Fab fragments (fragmented antigen binding) are the regions of an antibody expressed and secreted in E.coli cells. For the estimation of the dimensions of the fermenter empirical equations and rules of thumbs were exploited. The dimensions and the fermenter properties like the diameter, the height as well as the impellers speed, the baffles position and the driveshaft size were estimated on a scale- down model. The material used for the fabrication of the fermenter, the head end, the ports and the pipes is stainless steel to resist corrosion and maintain a clean and sterile vessel. The diameter of the vessel is 0.8m and the height is 2m.
The thickness of the wall is 6mm with a 0.15 m jacket surround it. The impeller’s speed is 3.3 m/s and the impeller used is Ruston Turbine four blades, suitable for the growth of E.coli cells. Therefore all the power requirements were satisfied ensuring the viability of the design. Five spray balls were found to be enough for a sufficient cleaning of the fermenter with their 360° coverage and placed on the inside of the torispherical head in a cross shape. Then the flowrate in the pipes was predicted enabling the calculation of the diameter. Basically all the information specified for every single part of the fermenter was taken from recommendations, advice from experts, theoretical predictions as well as engineering justifications. Last but not least emphasis is given on the importance of maintenance and annual shutdowns of the plant as well as regular checks to ensure its proper operation. The above graph, show the phases in the cell growth in a typical batch. The table, describes each phase and provides the specific growth rate for each phase. Reproduced from P.M.Doran (2006), pp:277
The above graphs, show the modeling of the growth kinetics and the typical profiles of biomass and substrate concentration during a batch process. Reproduced from Nielsen et al (2002), pp:247
Operation Cycle
The operation cycle for the fermenter involves start up, where process water is fed from the process water port and media is fed from the feed port into the vessel. The level of the liquid is measured using the level indicator probe. Once the required level is met then the ports close. Air is pumped through the air inlet to the sparger inside the fermenter.
The temperature is brought and maintained to the optimum temperature. There are two probes in the fermenter which are at the greatest range i.e. opposite sides of the fermenter as shown in the drawing. This is to ensure the distribution of heat is even and the average of both sensors should read 37°C. The pH is maintained at a desired value by the addition of acid and alkali. Two probes for measuring the pH are placed inside the fermenter at opposite ends to ensure there is good mixing as this would be indicated if there was little change between the two probes.
Once the optimum conditions have been achieved the innocculum is fed through the innocculum port. Antifoam from the antifoam port is added to avoid foam formation which reduces the efficiency of the process. The exhaust gases are released from the fermenter whilst in operation and the pressure is maintained. The cells are grown for a certain period of time and the process is regulated using a series of automatic control loops.
Finally there is shut down, where the fermentation batch ends and in this step all the broth is collected into the harvest port.
After each operation cycle the fermenter will have to be cleaned and sterilized in place. CIP will be performed using acid and base detergents which will be pumped through the cleaning solution ports into the 3 spray balls. SIP requires that the fermenter and all pipes are sterilized with steam at 121°C. The steam will come from the acid, base and antifoam ports. Regular and annual maintenance checks will have to be performed to ensure everything is running correctly and as efficiently as possible.
Maintenance
Typically, 3-5% of fermentations in an industrial plant are lost due to failure of sterilisation procedures. However in antibiotic fermentations like in this project, fewer than 2% of production scale antibiotic fermentations are lost through contamination by microorganisms or phage. (Doran, 1999)
Industrial fermenters are designed for in situ steam sterilisation under pressure. For effective sterilisation, all air in the vessel and pipe connections must be displaced by steam. After sterilisation, all nutrient medium and air entering the fermenter must be sterile.
Maintenance is required to ensure the fermentation process is achieving its goals, to identify any problems that may occur and ultimately keep the process working as efficiently as possible to save on costs. Maintaining the fermenter can significantly reduce the overall operating cost as well as minimise the risk of any hazards. For maintenance, the top of the fermenter can be removed to allow access of maintenance personnel for any repairs that may be required. For large vessels a domed construction at the top to allow access to personnel is less expensive and so this has been chosen in the design (Doran, 1999).
Once the design has been built and approved by British Standards 5500, the fermenter can then start to operate (Sinott, 1999). Maintenance is crucial and the fermenter must be monitored at all times. Most measurements can be made on-line through probes in the vessel which are sensors that detect changes in many variables such as temperature and pH. However, whilst monitoring some values there may always be a delay. For example, in a typical fermentation, the time scale for change in pH and dissolved-oxygen tension is several minutes to make sure they are all correctly calibrated and functioning properly and as efficiently as possible. This is to avoid any problems that may be caused because of a false reading. For example if the pH probe is not working properly and the pH is either too high or too low, then the cells would die and also the product may denature. Also possible blockages in pipes or broken valves must be checked regularly. Oxygen supply through bottom sparging should also be checked regularly, as cells die without it and that would mean a significant loss in time and money if the fermenter has been running for several days.
The fermenter requires annual shutdown to check for possible corrosion on the internal surface and also on the impellers. Corrosion may reduce the speed and efficiency of them impellers and the shaft too. This needs to be maintained at a high standard to ensure good mixing in the fermenter for growth of cells and product. Baffles also need to be checked for to see the level of corrosion. Also there may be possible fouling in the jackets and general piping problems, such as blockages and leakages, as well as overall contamination in the system. As previously mentioned in the design a 2mm allowance for corrosion had been added on the thickness of all equipment. The annual check would see how much all the equipment has been eroded and identify any problems to resolve them.
The aim of the Mechanical Drawing is to provide a detailed analysis of the construction and operation of the fermenter that is a technical way of presenting this piece of equipment. In this section the dimensions and materials of construction of a 1L fermenter were estimated. The fermenter is used for the growth of Escherichia coli cell culture for the production of therapeutic fragmented antibodies.
For the design project purpose several assumptions had to be made. The first one was the model taken for the scale- up process. This was the original 1L fermenter used for the pilot scale of the E.coli cells. Assuming that a 1000L fermenter operating with 75% space efficiency (0.75) and 1 marine impeller (di=dv/2). The vessel aspect ratio is 2.5 that is Ah/d= 2.5 and the fermenter is aerated at 0.75 vvm (that is 0.75 volumes of air per volume of liquid per minute). The gassed power requirement per m3 is Pg/VL= 1860 W/m3 as it was found from the model used for the scale- up. Usually the industrial motors are used to deliver 1-3 W/L (Lye et Baganz, 2007). Therefore the 1.86 W/L was chosen on the assumption that due to engineering cell modifications, the E.coli cells are not so robust and thus extremely high power inputs should be avoided. For this purpose instead of using a marine propeller a lighter choice was preferred since the Chinese Ovary cells are susceptible to mechanical damage (Farid, 2006) due to their large size and lack of rigid cell wall. Therefore the power input value must be kept to a minimum. A Rushton turbine four blade impeller is used as the axial flow type of impeller ensuring medium speed and ensuring medium growth rate but also good mixing.
Calculations for determination of the fermenter’s properties
The operating pressure of the vessel was assumed to be 3.5 bar and the design pressure 3.85 bar. The operating temperature for mammalian cells is 37°C (Lydersen et al, 1994) but the design temperature is 4- 121°C allowing the introduction of steam to clean the vessel.
Since the mammalian cells are very sensitive, an elevation in temperature e.g. 40°C can cause cell damage and death. Hence temperature probes allowing 〖_-^+〗0.5°C from the temperature set point as well as pH probe to maintain the appropriate range was considered.


 "
"



 Twitter
Twitter Facebook
Facebook UCL
UCL Flickr
Flickr YouTube
YouTube