| The enzymatic reaction catalysed by C2,3O is an ideal output signal for our engineered bacterial detector and it can also serve as a very useful reporter gene. Its characteristics can be exploited in a wide range of fields across the biological sciences, so it was one of the team's first candidates for further characterization. Qualitative and quantitative data have been gathered and presented. All further tests involving XylE transformed cells were quantified by measuring absorbance at 380nm wavelenth.
|
|
Catechol, the substrate of C2,3O, is colourless. However within seconds of its addition, the colonies/liquid cultures of XylE-expressing cells become yellow, indicating production of a product which absorbs light in the visible spectrum. A spectrophotometric assay was prepared, where the spectra of two cultures of E.coli (one XylE gene transformed and the other not) were compared on addition of 0.1mM Catechol substrate. The spectra (figure 1) showed that in XylE transformed cells, a broad peak appears at about 380nm. The absorbance at this particular wavelength is by the product of the C2,3O reaction which is called 2-hydroxymuconic semialdehyde and is what causes the yellow output.
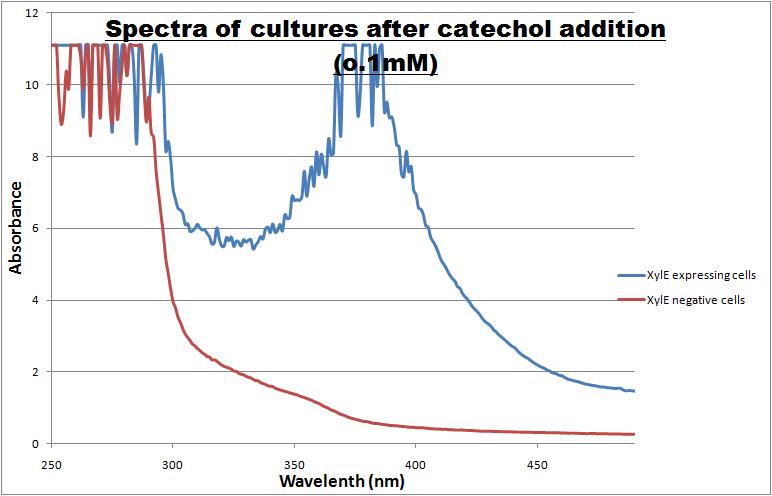
|
| Spectra of cultures of cells expressing the XylE and cells negative for the XylE gene. Note the broad peak in the spectra of Xyle transformed cells, which is centered around 380nm.
|
References
- Chromogenic identification of genetic regulatory signals in Bacillus subtilis based on expression of a cloned Pseudomonas gene. Zukowski, M.M., Gaffney, D.F., Speck, D., Kauffmann, M., Findeli, A., Wisecup, A., Lecocq, J.P. Proc. Natl. Acad. Sci. U.S.A. (1983)
|
 "
"





