Team:USTC Software/Transfer function of Part:BBa F2620
From 2010.igem.org
| Line 1: | Line 1: | ||
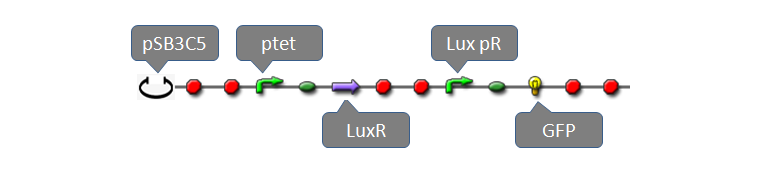
| - | Part:BBa_F2620 had been fully characterized with data in partsregistry [http://partsregistry.org/Part:BBa_F2620 BBa_F2620] page. It is a composite biobrick with three individual biobricks: pTetR (R0040), LuxR(C0062) and lux pR. Without AHL, transcriptional level of lux pR is extremal low because of lack of activator, LuxR-AHL dimer. If we add AHL into the system within a very short time, expression of mature GFP is expected to increase significantly. To measure behaviors of the system consisting of BBa_F2620 using our software tool, we construct a system using plasmid pSB3C5 and Part:BBa_F2620 as well as a reporter gene: | + | <font size="+1">Part:BBa_F2620</font> had been fully characterized with data in partsregistry [http://partsregistry.org/Part:BBa_F2620 BBa_F2620] page. It is a composite biobrick with three individual biobricks: pTetR (R0040), LuxR(C0062) and lux pR. Without AHL, transcriptional level of lux pR is extremal low because of lack of activator, LuxR-AHL dimer. If we add AHL into the system within a very short time, expression of mature GFP is expected to increase significantly. To measure behaviors of the system consisting of BBa_F2620 using our software tool, we construct a system using plasmid pSB3C5 and Part:BBa_F2620 as well as a reporter gene: |
[[Image:USTCs_luxr_gfp_assembly.PNG|700px|center]] | [[Image:USTCs_luxr_gfp_assembly.PNG|700px|center]] | ||
| Line 12: | Line 12: | ||
[[Image:USTC_s_dose_response.PNG|700px|center]] | [[Image:USTC_s_dose_response.PNG|700px|center]] | ||
| + | ---- | ||
| + | <font size="+1">A similar work</font> to measure system response of luxr-plux to inducer AHL was done by [https://2009.igem.org/Team:USTC USTC 2009 iGEM team]. Besides measurement of dose response of GFP stable concentrations following addition of AHL, they construct four constitutive promoters, [http://partsregistry.org/wiki/index.php/Part:BBa_K176026 BBa_K176026], [http://partsregistry.org/wiki/index.php/Part:BBa_K176126 BBa_K176126], [http://partsregistry.org/wiki/index.php/Part:BBa_K176128 BBa_K176128], [http://partsregistry.org/wiki/index.php/Part:BBa_K176130 BBa_K176130], and quantitatively measured their effects to the response curves. The construction is modified by replacing pTet with the four promoters. Since there are no tetR protein existed in the system, we keep lux pR unchanged (it is equivalent to use pLux/Tet hybrid promoter). | ||
| - | + | The results is shown for each: | |
Revision as of 03:03, 25 October 2010
Part:BBa_F2620 had been fully characterized with data in partsregistry BBa_F2620 page. It is a composite biobrick with three individual biobricks: pTetR (R0040), LuxR(C0062) and lux pR. Without AHL, transcriptional level of lux pR is extremal low because of lack of activator, LuxR-AHL dimer. If we add AHL into the system within a very short time, expression of mature GFP is expected to increase significantly. To measure behaviors of the system consisting of BBa_F2620 using our software tool, we construct a system using plasmid pSB3C5 and Part:BBa_F2620 as well as a reporter gene:
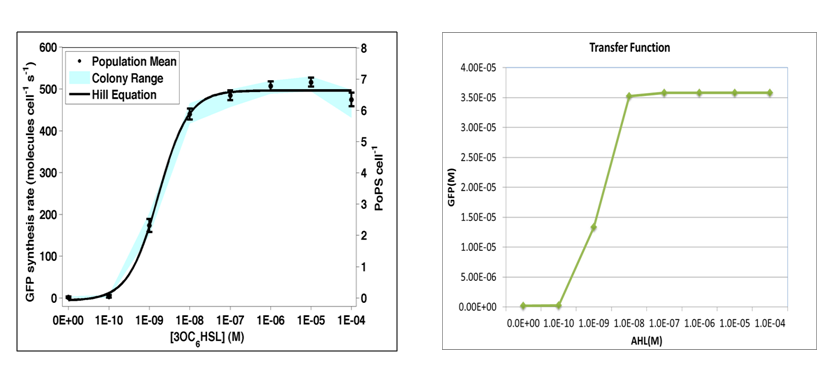
where we use two consecutive terminators to indicate a complete termination. Time course simulation was performed to generate transfer function of stable GFP concentrations versus AHL concentration. It is of high consistence with experiment done by Haseloff Lab, MIT:
where GFP concentration is directly proportional to its synthetic rate. In our simulation, we add AHL of concentration ranging from 1E-10 to 1E-5 M (increasing by order of magnitude) to the reactor within one minute. Details of modeling are described here.
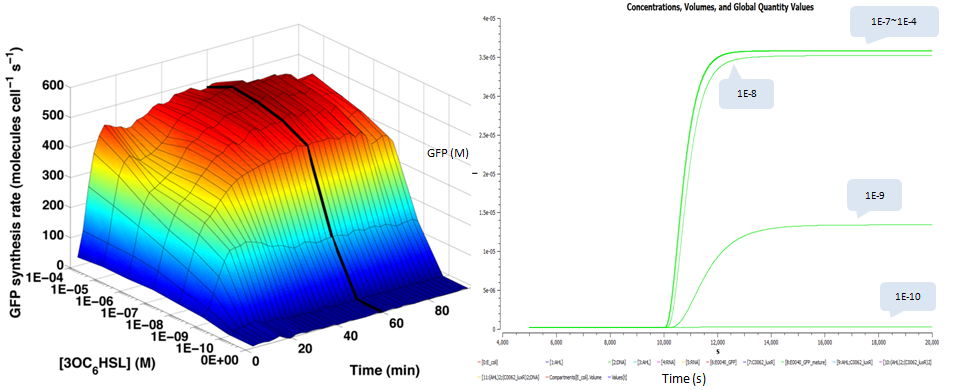
We also plot time and dose response measurements of GFP stable concentration following addition of AHL. We choose the same AHL concentrations as done in testing the transfer function and plot their dynamic curves of GFP:
A similar work to measure system response of luxr-plux to inducer AHL was done by USTC 2009 iGEM team. Besides measurement of dose response of GFP stable concentrations following addition of AHL, they construct four constitutive promoters, BBa_K176026, BBa_K176126, BBa_K176128, BBa_K176130, and quantitatively measured their effects to the response curves. The construction is modified by replacing pTet with the four promoters. Since there are no tetR protein existed in the system, we keep lux pR unchanged (it is equivalent to use pLux/Tet hybrid promoter).
The results is shown for each:
 "
"