Team:UNIPV-Pavia/Calendar/September/settimana4
From 2010.igem.org
(→September, 23rd) |
(→September, 23rd) |
||
| (42 intermediate revisions not shown) | |||
| Line 37: | Line 37: | ||
==September, 20th== | ==September, 20th== | ||
| - | Screening PCR on 6 colonies picked from I55 was performed. | + | Screening PCR on 6 colonies picked from I55 was performed. Results were the following: |
| - | [[Image:UNIPV1020_09_10_I55.jpg|thumb| | + | [[Image:UNIPV1020_09_10_I55.jpg|thumb|200px|center| I55-1..6.]] |
| - | - | + | I55-3 seems to be ok. |
Inoculum of I52, I53, I55, I56 and I57 in LB+Amp for MiniPrep of the following day | Inoculum of I52, I53, I55, I56 and I57 in LB+Amp for MiniPrep of the following day | ||
| Line 61: | Line 61: | ||
| ''Culture'' || ''Kind'' || ''Final reaction volume (ul) '' || ''DNA (ul)'' || ''H20 (ul)'' || ''Enzyme 1 (ul)'' || ''Enzyme 2 (ul)'' || ''Buffer H (ul)'' | | ''Culture'' || ''Kind'' || ''Final reaction volume (ul) '' || ''DNA (ul)'' || ''H20 (ul)'' || ''Enzyme 1 (ul)'' || ''Enzyme 2 (ul)'' || ''Buffer H (ul)'' | ||
|- | |- | ||
| - | | I52 || | + | | I52 || Vector|| 25 || 10,8 || 9,7 || 1 EcoRI || 1 XbaI || 2,5 |
|- | |- | ||
| - | | I53 || | + | | I53 || Vector|| 25 || 8,5 || 12 || 1 EcoRI || 1 XbaI || 2,5 |
|- | |- | ||
| - | | I55 || Screening || 25 || 14 || 6,5 || 1 EcoRI || 1 SpeI || 2,5 | + | | I55 || Insert/Screening|| 25 || 14 || 6,5 || 1 EcoRI || 1 SpeI || 2,5 |
|- | |- | ||
| - | | I56 || | + | | I56 || Insert/screening|| 25 || 10 || 10,5 || 1 XbaI || 1 PstI || 2,5 |
|- | |- | ||
| - | | I57 || | + | | I57 || Vector|| 25 || 9,7 || 10,8 || 1 EcoRI || 1 XbaI || 2,5 |
|- | |- | ||
| - | | I61 || | + | | I61 || Vector|| 25 || 6,8 || 13,7 || 1 EcoRI || 1 XbaI || 2,5 |
|} | |} | ||
| Line 77: | Line 77: | ||
Gel run/cut of samples | Gel run/cut of samples | ||
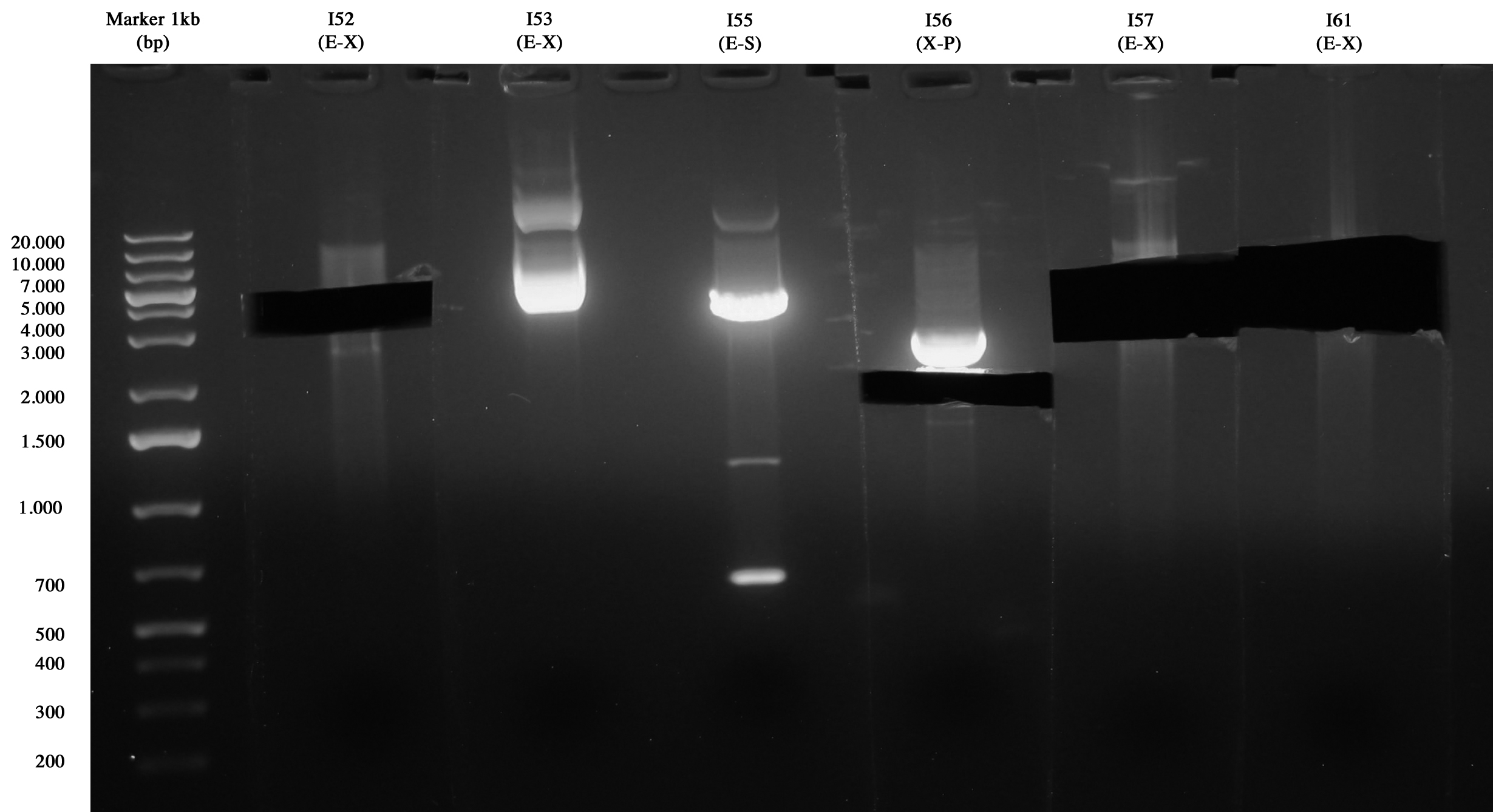
| - | [[Image:UNIPV1021_09_10.jpg|thumb| | + | [[Image:UNIPV1021_09_10.jpg|thumb|200px|center| I52(E-X), I53(E-X), I55(E-S), I56(X-P), I57(E-X), I61(E-X).]] |
| - | As you can see only I52, I56, I57 and I61 | + | As you can see only I52, I56, I57 and I61 run correctly (I55 was wrong) so gel extraction was performed on these samples: |
* I52 = 29,4 ng/ul | * I52 = 29,4 ng/ul | ||
| Line 88: | Line 88: | ||
We already had digested DNA so we could perform new ON ligations: | We already had digested DNA so we could perform new ON ligations: | ||
| - | * I62 = | + | * I62 = I39(S-P) + I59(X-P) |
| - | * I63 = | + | * I63 = I38(E-S) + I61(E-X) |
| - | * I64 = | + | * I64 = I58(E-S) + I52(E-X) |
* I65 = I39(S-P) + I56(X-P) | * I65 = I39(S-P) + I56(X-P) | ||
| - | * I66 = | + | * I66 = I60(E-S) + I57(E-X) |
---- | ---- | ||
| - | + | Tecan Test on self-inducible promoters was performed on prepared samples with a different protocol that is to bring the cultures to a known OD. | |
<div align="right"><small>[[#indice|^top]]</small></div> | <div align="right"><small>[[#indice|^top]]</small></div> | ||
| Line 100: | Line 100: | ||
==September, 22nd== | ==September, 22nd== | ||
| - | Trasformation of | + | Trasformation of ligations I62, I63, I64, I65 and I66 into ''E.coli'' TOP10. |
| + | |||
| + | ---- | ||
| + | |||
MiniPrep and quantification of: | MiniPrep and quantification of: | ||
| Line 122: | Line 125: | ||
Gel run of samples was performed | Gel run of samples was performed | ||
| + | [[Image:UNIPV10_22_09_10_MyYeast_screening.jpg|thumb|200px|center| MyYeast-1/2/3 (EcoRI-NsiI digest).]] | ||
| - | + | None of samples was positive. | |
| - | + | ||
| - | + | ||
| - | + | ||
Glycerol stocks of MyYeast1, MyYeast2, MyYeast3 was performed. | Glycerol stocks of MyYeast1, MyYeast2, MyYeast3 was performed. | ||
| Line 136: | Line 137: | ||
==September, 23rd== | ==September, 23rd== | ||
| - | Digestion of yesterday are repeted (because | + | Digestion of yesterday are repeted (because none of them were postitive) as follows: |
{| border="1" align='center' | {| border="1" align='center' | ||
| Line 162: | Line 163: | ||
Gel run of samples was performed | Gel run of samples was performed | ||
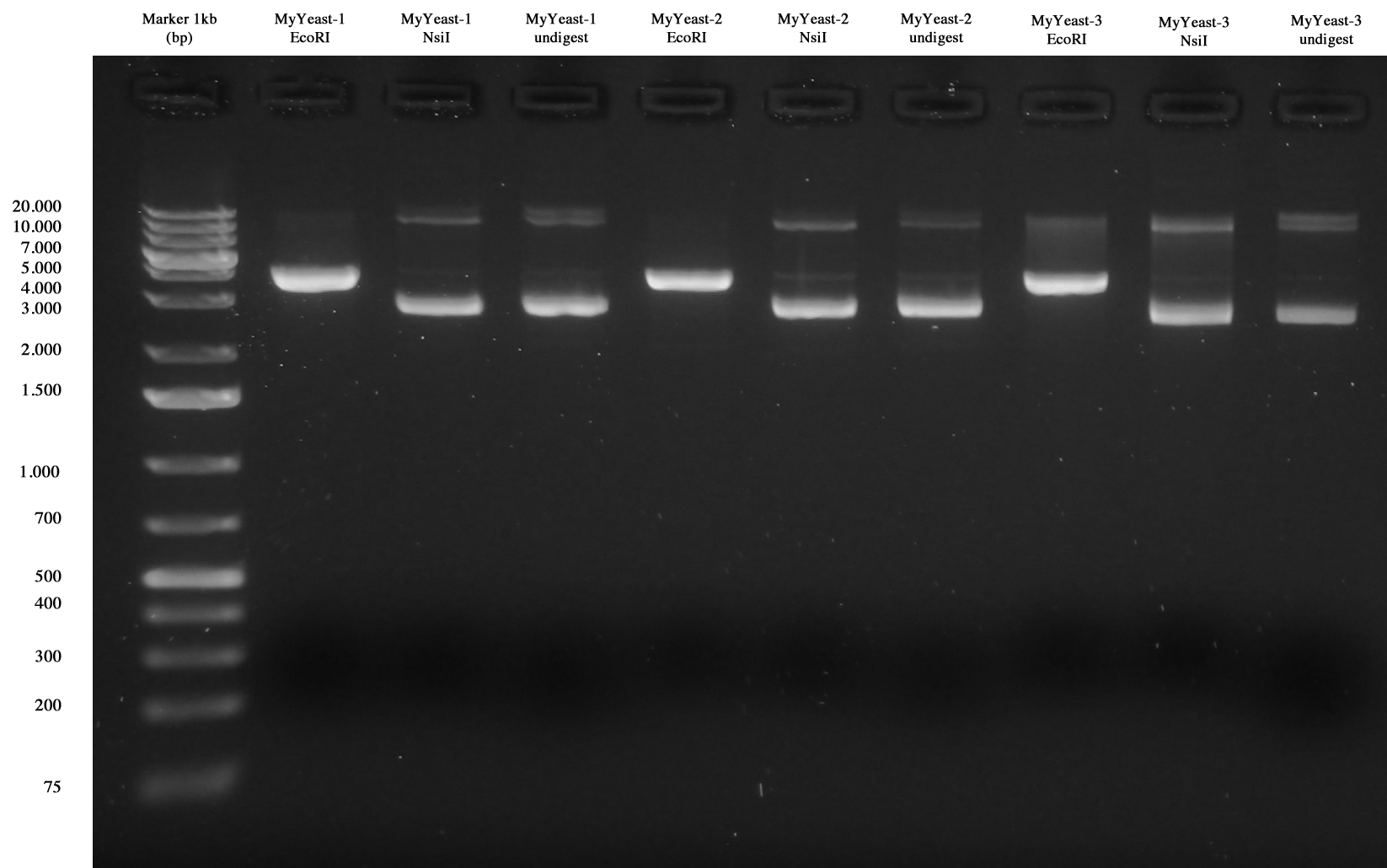
| + | [[Image:UNIPV10_23_09_10_MyYeast_digest.jpg|thumb|200px|center|MyYeast-1/2/3 digest.]] | ||
All samples seem to be positive!! | All samples seem to be positive!! | ||
| Line 167: | Line 169: | ||
---- | ---- | ||
| - | Inoculum | + | Inoculum for three colonies from I62, I63, I64, I65 agar plates into 5ml LB+Amp. |
<div align="right"><small>[[#indice|^top]]</small></div> | <div align="right"><small>[[#indice|^top]]</small></div> | ||
==September, 24th== | ==September, 24th== | ||
| + | Glycerol stocks was performed for | ||
| + | * I62-1,2,3 | ||
| + | * I63-1,2,3 | ||
| + | * I64-1,2,3 | ||
| + | * I65-1,2,3 | ||
| + | * I66-1,2,3 | ||
| - | + | MiniPrep and Nanodrop quantification : | |
| + | * I62-1 : 35,1 ng/ul | ||
| + | * I62-2 : 27,8 ng/ul | ||
| + | * I62-3 : 63,8 ng/ul | ||
| + | * I63-1 : 195,3 ng/ul | ||
| + | * I63-2 : 68,3 ng/ul | ||
| + | * I63-3 : 148,7 ng/ul | ||
| + | * I64-1 : 97,8 ng/ul | ||
| + | * I64-2 : 171,8 ng/ul | ||
| + | * I64-3 : 146,3 ng/ul | ||
| + | * I65-1 : 69,7 ng/ul | ||
| + | * I65-2 : 44 ng/ul | ||
| + | * I65-3 : 294,3 ng/ul | ||
| + | * I66-1 : 292,8 ng/ul | ||
| + | * I66-2 : 198 ng/ul | ||
| + | * I66-3 : 284,8 ng/ul | ||
| - | + | Digestion for screening : | |
| + | * I62-1,2,3 (E-P) | ||
| + | * I63-1,2,3 (E-P) | ||
| + | * I64-1,2,3 (E-P) | ||
| + | * I65-1,2,3 (E-P) | ||
| + | * I66-1,2,3 (E-P) | ||
| + | Gel run of samples was performed | ||
| + | |||
| + | [[Image:UNIPV10_24_09_10_screening_I62-3-4-5-6.jpg|thumb|200px|center| Screening for I62/3/4/5/6 (E-P) digested.]] | ||
| + | |||
| + | As you can see I62-1/2/3 were all negative; I63-1/2/3, I64-1/2/3, I65-3, I66-1/2/3 were positive. So we made glycerol stocks for I63-1, I64-2, I65-3, I66-1 and stored them at -80°C. We stored their DNA in -20°C, too. | ||
| + | ---- | ||
| + | Dilution 1:50 of I47, I48, I49, GFP in LB+Amp+Cm12,5 and S1 in LB+Cm12,5. | ||
| + | |||
| + | All cultures were grow up to OD=0,1 of Nanodrop. They were then induced with HSL (lysis induction) 100nM | ||
<div align="right"><small>[[#indice|^top]]</small></div> | <div align="right"><small>[[#indice|^top]]</small></div> | ||
| + | ==September, 26th== | ||
| + | |||
| + | Inoculum of <partinfo>BBa_K173000</partinfo>, I47, I48, I49, <partinfo>BBa_B0031</partinfo> into 3 ml LB+Amp for tomorrow TECAN test. Pick of six colonies from I66 agar plate (I66-A/B/C/D/E/F) and inoculum into 3 ml LB+Amp for screening thorough E-P digest. All colonies were let grow ON at 37°C, 220 rpm. | ||
| + | |||
| + | <div align="right"><small>[[#indice|^top]]</small></div> | ||
<!-- table previous next week --> | <!-- table previous next week --> | ||
<br><br> | <br><br> | ||
Latest revision as of 19:14, 27 October 2010
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"