|
|
| Line 20: |
Line 20: |
| | | | |
| | ====Week 6==== | | ====Week 6==== |
| | + | |
| | + | |
| | <html> | | <html> |
| | <table width="850px" border="0"> | | <table width="850px" border="0"> |
| Line 36: |
Line 38: |
| | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Friday</b> | | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Friday</b> |
| | </td> | | </td> |
| - | </tr>
| + | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Saturday</b> |
| - | <tr>
| + | |
| - | <td style="background-color:#FFCC66;width:100px;text-align:center;font-family: helvetica, arial, sans-serif;color:#555555;"><b>Morning</b>
| + | |
| - | </td>
| + | |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| + | |
| - | <ul>
| + | |
| - | <li></li>
| + | |
| - | </ul>
| + | |
| - | </td>
| + | |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| + | |
| - | <ul>
| + | |
| - | <li></li>
| + | |
| - | </ul>
| + | |
| - | </td>
| + | |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| + | |
| - | <ul>
| + | |
| - | <li></li>
| + | |
| - | </ul>
| + | |
| - | </td>
| + | |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| + | |
| - | <ul>
| + | |
| - | <li></li>
| + | |
| - | </ul>
| + | |
| - | </td>
| + | |
| - | <td style="background-color:#e7e7e7;height:100px;width:150px;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| + | |
| - | <ul>
| + | |
| - | <li></li>
| + | |
| - | </ul>
| + | |
| - | </td>
| + | |
| - | </tr>
| + | |
| - | <tr>
| + | |
| - | <td style="background-color:#FFCC66;text-align:center;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;"><b>Afternoon</b>
| + | |
| - | </td>
| + | |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| + | |
| - | <ul>
| + | |
| - | <li></li>
| + | |
| - | </ul>
| + | |
| - | </td>
| + | |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;font-family: helvetica, arial, sans-serif;color:#555555;">
| + | |
| - | <ul>
| + | |
| - | <li></li>
| + | |
| - | </ul>
| + | |
| - | </td>
| + | |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| + | |
| - | <ul>
| + | |
| - | <li></li>
| + | |
| - | </ul>
| + | |
| - | </td>
| + | |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| + | |
| - | <ul>
| + | |
| - | <li></li>
| + | |
| - | <li></li>
| + | |
| - | <li></li>
| + | |
| - | </ul>
| + | |
| - | </td>
| + | |
| - | <td style="background-color:#e7e7e7;text-align:top;font-family: helvetica, arial, sans-serif;color:#555555;">
| + | |
| - | <ul>
| + | |
| - | <li></li>
| + | |
| - | <li></li>
| + | |
| - | </ul>
| + | |
| - | </td>
| + | |
| - | </tr>
| + | |
| - | </table>
| + | |
| - | </html>
| + | |
| - | | + | |
| - | <html>
| + | |
| - | <table width="850px" border="0">
| + | |
| - | <tr>
| + | |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;">
| + | |
| - | <b>Day</b>
| + | |
| - | </td>
| + | |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Monday</b>
| + | |
| - | </td>
| + | |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Tuesday</b>
| + | |
| - | </td>
| + | |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Wednesday</b>
| + | |
| - | </td>
| + | |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Thursday</b>
| + | |
| - | </td>
| + | |
| - | <td style="background-color:#FFFF99;text-align:center; font-family: helvetica, arial, sans-serif;color:#555555;"><b>Friday</b> | + | |
| | </td> | | </td> |
| | </tr> | | </tr> |
| Line 139: |
Line 62: |
| | </ul> | | </ul> |
| | </td> | | </td> |
| | + | <td style="background-color:#e7e7e7;height:100px;width:200px;text-align:top;"> |
| | + | </td> |
| | </tr> | | </tr> |
| | | | |
| Objectives
|
|
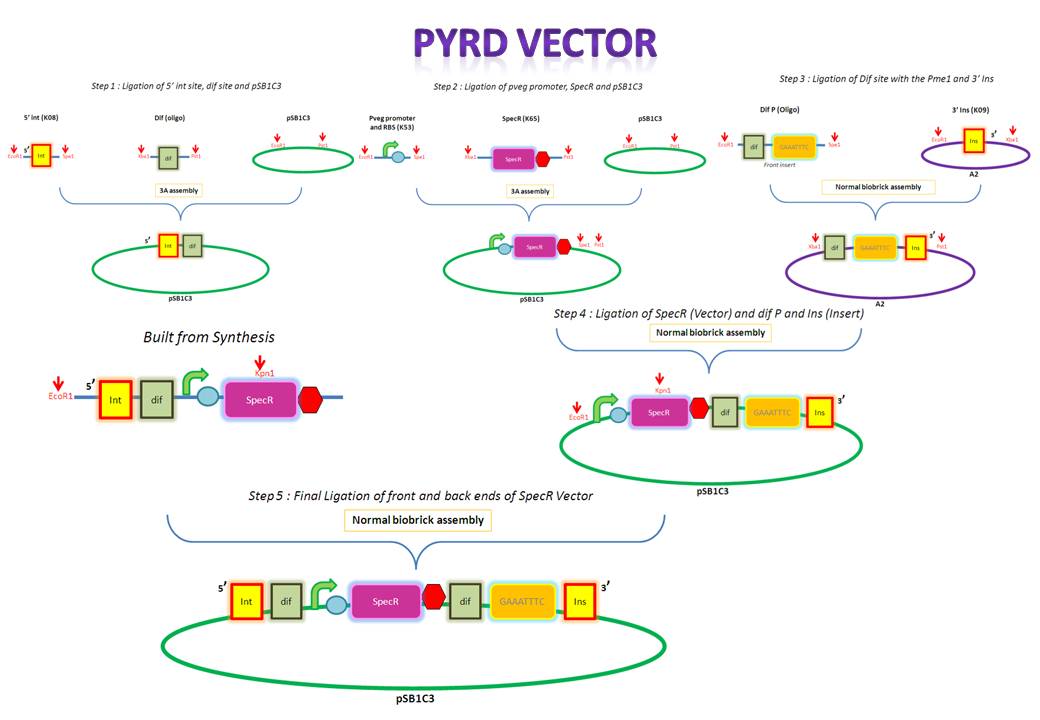
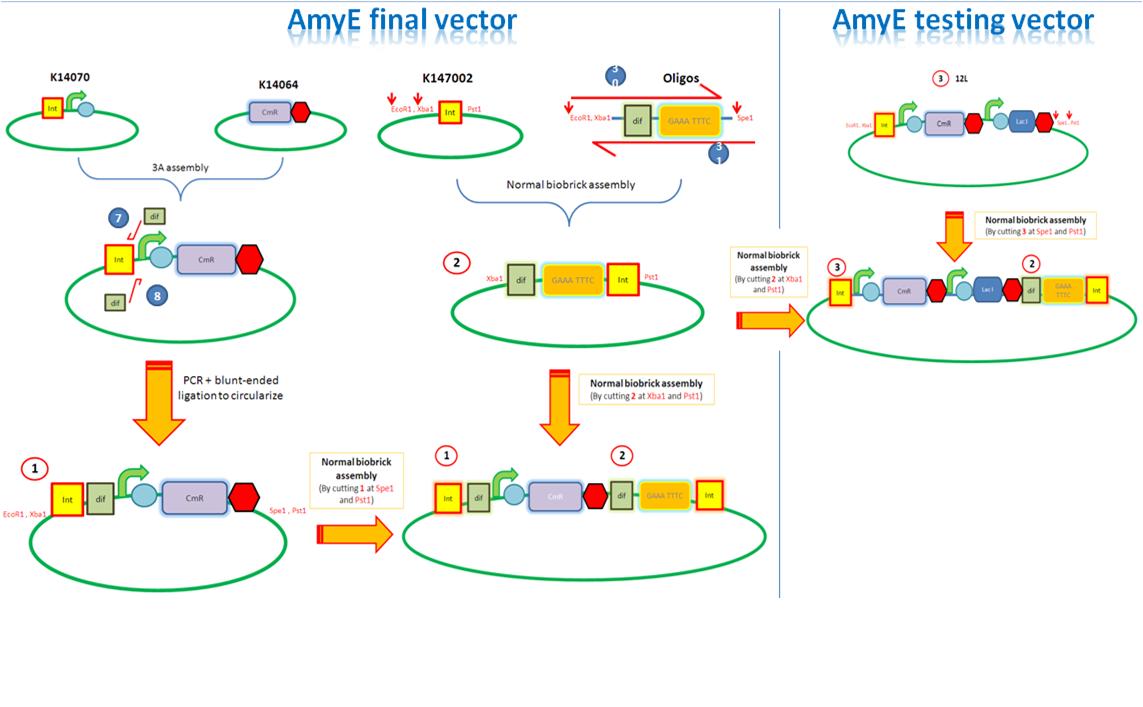
We are assembling the AmyE and PyrD vectors in order to transform B. Subtilis with our parts. Once completed, these vectors will be reusable and can then be used to introduce any relevant piece of DNA directly into B.subtilis genome. The vectors will be used both for the final assembly and for testing constructs.
|
Week 6
|
Day
|
Monday
|
Tuesday
|
Wednesday
|
Thursday
|
Friday
|
Saturday
|
| Morning
|
|
|
|
|
- Restriction digest of 5' dif XP with XbaI and PstI
|
|
Afternoon
|
|
|
|
|
Start assembly of PyrD vector
- Overnight annealing of 5' Ins ( synthesized oligos )
|
- Gel analysis of resultant products from 5' dif XP digest
- PCR purification of cut 5' dif XP
|
Thursday, August 12
- Annealing the forward and reverse strands of the dif XP oligo
The forward and reverse strands of the 5' dif site with XbaI and PstI restriction sites on either side have been synthesized separately. The synthesized fragments arrive in solid powder form. These were immediately diluted in ddH2O to obtain a stock concentration of 1 ng/ul. They were then allowed to anneal together by first heating them to 95 degs for denaturation and allowing them to cool down and anneal overnight.
Friday, August 13
- Restriction digest of dif XP
After annealing the two strands, the oligo was cut with XbaI and PstI to obtain overhangs that would later ligate with the compatible overhangs of a cut vector.
- PCR purification of the cut dif XP
The cut oligo was PCR purified in order to get rid of any contaminants. PCR purification gets rid of short pieces of DNA which are less than about 40 base pairs.
Week 7
|
Day
|
Monday
|
Tuesday
|
Wednesday
|
Thursday
|
Friday
|
| Morning
|
- Restriction digestion of 5' ins [K143008] (using Eco and Spe)
- PCR amplification of vector backbone pSB1C3
|
- Gel analysis and extraction of 5' ins
- Gel purification of 5' ins
- Restriction digestion of pSB1C3 (using Eco and Pst)
- PCR purification of pSB1C3 after digestion
- Gel analysis of 5' ins, dif and pSB1C3 to work out ratios for ligation
- Restriction digestion of pveg promoter and RBS [K143053] (using Eco and Spe)
- Restriction digetion of SpecR-T [K143065] (using Xba and Pst)
|
- Replica plating and colony PCR of transformed colonies (containing 5' dif in pSB1C3)
- Gel extraction and PCR purification of pveg and SpecR-T in preparation for ligations this afternoon
- Gel analysis of pveg , Spec-T and pSB1C3 in order to work out ratios for the ligation
|
- Transformation of overnight ligations: 5' ins, dif with pSB1C3 and pveg, SpecR-T and pSB1C3
|
- Replica plating and colony PCR of: 5' ins, dif with pSB1C3 and pveg, SpecR-T and pSB1C3
|
| Afternoon
|
- PCR purification of PSB1C3 vector
|
- Ligation of 5' ins and dif with pSB1C3 - a bench ligation (1 hour) and an overnight ligation were set up
- Transformation of E.Coli with the bench ligated products
- Gel analysis and extraction of pveg and SpecR-T
|
- Overnight ligation of pveg and SpecR-T with pSB1C3
|
|
- Gel analysis of colony PCR products from the transformations
- Overnight annealing of diff PES (Synthesized oligo)
|
Monday, August 16
- Restriction digest of 5' ins [K143008]
K143008 is the 5' integration site for the PyrD vector. This will be used as a front insert together with the dif XP for the pSB1C3 vector.
- PCR amplification of pSB1C3
The pSB1C3 vector backbone from the registry was amplified with the use of SB3 and SB2a primers. Submission of parts to the registry requires them to be in a pSB1C3 vector therefore any parts to be submitted will be inserted into this vector.
- PCR purification of pSB1C3
The PCR amplified vector was purified in order to get rid of any contaminants. For example, short pieces of DNA like the primers.
Tuesday, August 17
- Gel extraction and purification of 5' ins ES
The digested 5' ins was first analyzed on the gel to verify it's size and then extracted for purification. The 5' ins was gel purified in order to extract only the relevant piece of DNA.
- Restriction digest of pSB1C3
The pSB1C3 vector was digested so that it would contain compatible overhangs for ligation with inserts.
- PCR purification of pSB1C3 EP
The digested pSB1C3 was re-purified in order to get rid of any contaminant DNA that arose during the digestion.
- Restriction digests of pveg and Spec-T
pveg (promoter and RBS) and Spec-T (Spectinomycin with a terminator) were digested in preparation for 3A assembly.
- Ligation of 5' ins (ES) and dif (XP) with pSB1C3 (EP)
The digested 5' ins and dif (the front inserts) were ligated overnight with pSB1C3 (the vector). A bench ligation and an overnight ligation were set up.
- Transformation of E.Coli with bench ligate
E.Coli was transformed via the chemical method using the bench ligate.
- Gel extraction and purification of pveg and Spec-T
Since these are both inserts they were gel extracted and purified. PCR purification is not carried out for inserts since they are small and would therefore be lost during the process.
Wednesday, August 18
- Replica plating and colony PCR of 5' ins and dif in pSB1C3 (bench ligation)
- Ligation of pveg (ES) and SpecR-T (XP) with pSB1C3 (EP)
pveg and SpecR-T (the front inserts) were ligated overnight with pSB1C3 (the vector).
Thursday, August 19
- Transformation of E.Coli with the overnights ligates
- 5' ins and dif in pSB1C3
- pveg and SpecR-T in pSB1C3
Friday, August 20
- Replica plating and colony PCR of both transformations from yesterday.
- Annealing the forward and reverse strands of the dif P ES oligo
The forward and reverse strands of the 3' dif Pme1 sites with XbaI and PstI restriction sites on either side have been synthesized separately. The synthesized fragments arrive in solid powder form. These were immediately diluted in ddH2O to obtain a stock concentration of 1 ng/ul. They were then allowed to anneal together by heating them to 95 degs for denaturation and allowing them to cool down and anneal overnight.
Week 8
|
Day
|
Monday
|
Tuesday
|
Wednesday
|
Thursday
|
Friday
|
| Morning
|
- Set up overnight ligations for standard assembly (BBA) and 3A cloning (3A) of dif P
- Restriction digestion of 3' ins in the pSB1AK3 vector ( Using Eco and Xba) for BBA
- PCR purification of 3' ins in AK3 ( Using Eco and Xba) for BBA
- Set up 5 ml culture of pveg, SpecR-T in pSB1C3 from colony 1 of replica plate and kept in shaking incubator at 37 degrees
|
- Restriction digestion of 3' ins in the AK3 vector ( Using Xba and Pst) for 3A
|
- Overnight ligation of dif P and 3' ins with pSB1C3
- Transformations using the overnight ligations showed a lot of background. Therefore we set up ligations for K02 and K09 using 3A cloning. The results will show if this method is preferable due to less background.
|
- Replica plating of transformed colonies having dif P with 3' ins in AK3 -45 sigle colonies were plated
- The first 15 of the above colonies were used for colony PCR reactions using dif PES Fwd and pSB Rev
|
- Gel analysis of colony PCR from yesterday (first 15 replica plated colonies
- Set up further colony PCR reactions for the same 15 colonies using pSB Fwd and pSB Rev primers
|
- Miniprep of 4 overnight cultures - diff P with 3' ins in AK3; colonies 4,5,7 & 9
|
| Afternoon
|
- Gel analysis of PCR purified 3' ins in AK3 and gel purified 3' diff P to work out ratios for the liagation
- Dephosphorylation of digested AK3 vector with 3' ins
- Overnight ligation of dif P with 3' ins in AK3 (insert and vector) and AK3 containing 3' ins vector only
- Set up 100 ml culture of pveg and Spec-T in pSB1C3 by transferring 5 ml culture set up in the morning and kept in shaking incubator at 37 degrees
|
- Transformation of E.Coli with ligates from yesterday in AmpR;dif P with 3' ins in AK3 (insert and vector) and AK3 containing 3' ins vector only
- Gel extraction of 3' ins (now our insert) described this morning for 3A
- Gel analysis of gel extracted dif P and 3' ins (inserts) and pSB1C3 (vector) to work out ratios for the ligation
- Midiprep of pveg, SpecR-T in pSB1C3 from colony
|
- Gel analysis of gel extracted dif P and 3' ins (inserts) and pSB1C3 (vector) to work out ratios for the ligation
- pveg, SpecR-T in pSB1C3 midiprep sent for sequencing
|
- Set up overnight 5 ml cultures containing dif P with 3' ins in AK3 for miniprep tomorrow - 4 cultures were set up by looking at the gel this morning; 2 positive looking (4 & 7), 1 negative (5) and one containing nothing (9
|
- Diagnostic digests of minipreps containing dif P with 3' ins in AK3 - Two digests : One with Spe & Pst and other with Xba & Spe
|
|
Week 9
|
Day
|
Monday
|
Tuesday
|
Wednesday
|
Thursday
|
Friday
|
| Morning
|
- Gel analysis of Mini preps and diagnostic digests from Saturday - Colony 4 looked best
|
- Midiprep of Colony 4 - concentration 110 ng/ul
|
- Gel purification of insert (35 ul)
- Gel analysis of vector and insert to work out ratios for ligations
|
- Transformation with overnight ligations
|
- The transformations were highly successful!! The Vector only plate showed no colonies and the Insert & Vector plate showed many individual colonies
- 10 individual colonies were replica plated and used for colony PCR
|
Afternoon
|
- Set up 200 ml overnight culture of colony 4 (containing Dif P and K09) for midiprep tomorrow
|
- Digests set up for Vector (SpecR) with Spe & Pst and Insert (dif P & K09)with Xba & Ps
- PCR purification of vetor (35 ul)
- Gel extraction of insert after gel analysis (35 ul)
|
- Dephosphorylation of vector
- Set up two overnight ligations; Vector & Insert and Vector only
|
- Both ligations (Insert & Vector and Vector only) were plated in CmR and incubated overnight
|
- Gel analysis of colony PCR products
|
Week 10
|
Day
|
Monday
|
Tuesday
|
Wednesday
|
Thursday
|
Friday
|
| Morning
|
- Colony PCR of 10 transformed C-Spec colonies
- Set up 5ml culture of N-Spec in KanR
|
- Miniprep of C-Spec colonies 2, 5 & 9 in SpecR and CmR
- Digest of minipreps; double digest (Eco and Spe) and triple digest (PmeI, Eco and Spe)
- Gel analysis of midiprepped undigested and digested C-Spec
|
- Miniprep of C-Spec colonies 2, 5 & 9 in SpecR only and CmR only
- Digest of minipreps; double digest (Eco and Spe) and triple digest (PmeI, Eco and Spe)
- Gel analysis of midiprepped undigested and digested C-Spec
|
- Start midiprep of C-Spec from colony 2 (isopropanol added elute was refrigerated for 4 hrs)
- Measure concentration of midiprepped N-Spec (732 ng/ul)
|
- Dilution of N-Spec and C-Spec (4x)
- Double digest of N-Spec and C-Spec (Eco and KpnI)
- Gel analysis of diluted undigested and double digested Specs
|
Afternoon
|
- Gel analysis of colony PCR
- Set up 5 ml cultures of C-Spec colonies 2,5 & 9 in SpecR & CmR
- Transfer 5 ml culture to 100 ml culture in KanR (incubated overnight)
|
- Start midiprep of N-Spec (isopropanol added elute refrigerated overnight)
- Set up 5 ml cultures for C-Spec from colonies 2,5 & 9 (in SpecR only and CmR only)
|
- Continue midiprep of N-Spec (by ethanol preciptation)
- Set up a 500 ml overnight culture (with SpecR) of C-Spec from colony 2 for a midiprep tomorrow
|
- Continue midiprep of C-Spec (by ethanol precipitation)
- Digest of midiprepped N-Spec and C-Spec (Eco and Spe)
- Gel analysis of undigested and digested (Eco and KpnI) Specs
|
- Single digest of diluted C-Spec (Eco only)
- Gel analysis of diluted undigested, single digested (Eco only) and double digested (Eco and KpnI)
|
Week 11
|
Day
|
Monday
|
Tuesday
|
Wednesday
|
Thursday
|
Friday
|
| Morning
|
- Set up digests of N-Spec and C-Spec (Eco and KpnI)- (CD 1)
- Gel analysis and extraction of both N and C Specs
|
- Gel purification of N and C Specs from CD 2
- Run both Specs from each of the ligations on a gel
|
- Backbone PCR of pSB1C3 vector using PFU ultra buffer
- PCR purification of pSB1C3
|
- Backbone PCR of pSB1C3 using Barns buffer
- Replica plating in SpecR of transformed colonies from the insert and vector ligation and setting up 5 ml cultures in SpecR for transformed colonies
|
- Gel purification of pSB1C3
- Miniprep of 5 ml overnight cultures of colonies 1, 2 and 3
|
Afternoon
|
- Set up digests of N-Spec and C-Spec again, however, with a higher dilution of N-Spec - (CD 2)
- Gel analysis and extraction of N and C Specs
- Gel purification of CD 1
|
- Dephosphorylate vector (C-Spec) from CD 2
- Set up overnight ligations of Vector only (C-Spec) and Vector and Insert (N-Spec and C-Spec) for transformation
|
- Transformation of E.Coli with the two Spec final ligates - C-Spec (vector only) and N and C Specs (vector and insert)
- Gel analysis of purified pSB1C3
|
- Gel analysis and extraction of pSB1C3
|
- Test digest of final spec minipreps with Eco and Kpn1 and Eco and Spe
- Cloning digest of pSB1C3 with Eco and Pst
- Gel analysis of undigested and digested minipreps and digested pSB1C3
|
Week 12
|
Day
|
Monday
|
Tuesday
|
Wednesday
|
Thursday
|
Friday
|
| Morning
|
- Set up 5 ml culture for miniprep of C-Spec of colnies 1 and 2 from synthesis in AmpR
- Set up sequencing mixes for 3' dif P Ins in AK3, C-Spec minis from cultures 5 & 9 and final spec mini
|
- Start midiprep of C-Spec
- Miniprep of C-Spec cultures from yesterday
|
Start midiprep of C-Spec colonies again
|
- Cloning digests of C-Spec with Kpn1 and Pst and N-Spec with Eco and Kpn1
- Gel purification of the inserts
|
|
|
Afternoon
|
- Miniprep cultures of C-Spec have not grown well enough therefore they will be left overnight
- Set up 100 ml culture for midiprepping tomorrow
|
- Test digest C-Spec mini with ECo and KpnI
- Run test digest on gel, both minis from colonies 1 & 2 look good, therefore proceed to midiprep
- Continue with midiprep but no pellet after 45 minute spin
- Set up 100 ml cultures of colonies 1 & 2 for midiprep tomorrow
|
- Continue Midiprep of C-Spec, pellets obtained
- Transformation of E.Coli with the two Spec final ligates - C3 (vector only) and N and C Specs in C3 (vector and insert)
|
- Ligation gel with inserts; N-Spec (E & Kpn1) and C-Spec (Kpn1 & P) and Vector; pSB1C3 (E & P)
- Dephosphorylation of Vector and overnight ligation of vector alone and vector with inserts
|
- Transformation of E.Coli with the two Spec final ligates - C3 (vector only) and N and C Specs in C3 (vector and insert)
|
- Transformations failed, re-try next week!
|
Week 13
|
Day
|
Monday
|
Tuesday
|
Wednesday
|
Thursday
|
Friday
|
| Morning
|
- Set up 5 ml culture for miniprep of C-Spec of colnies 1 and 2 from synthesis in AmpR
- Set up sequencing mixes for 3' dif P Ins in AK3, C-Spec minis from cultures 5 & 9 and final spec mini
|
- Start midiprep of C-Spec
- Miniprep of C-Spec cultures from yesterday
|
Start midiprep of C-Spec colonies again
|
- Cloning digests of C-Spec with Kpn1 and Pst and N-Spec with Eco and Kpn1
- Gel purification of the inserts
|
|
|
Afternoon
|
- Miniprep cultures of C-Spec have not grown well enough therefore they will be left overnight
- Set up 100 ml culture for midiprepping tomorrow
|
- Test digest C-Spec mini with ECo and KpnI
- Run test digest on gel, both minis from colonies 1 & 2 look good, therefore proceed to midiprep
- Continue with midiprep but no pellet after 45 minute spin
- Set up 100 ml cultures of colonies 1 & 2 for midiprep tomorrow
|
- Continue Midiprep of C-Spec, pellets obtained
- Transformation of E.Coli with the two Spec final ligates - C3 (vector only) and N and C Specs in C3 (vector and insert)
|
- Ligation gel with inserts; N-Spec (E & Kpn1) and C-Spec (Kpn1 & P) and Vector; pSB1C3 (E & P)
- Dephosphorylation of Vector and overnight ligation of vector alone and vector with inserts
|
- Transformation of E.Coli with the two Spec final ligates - C3 (vector only) and N and C Specs in C3 (vector and insert)
|
- Transformations failed, re-try next week!
|
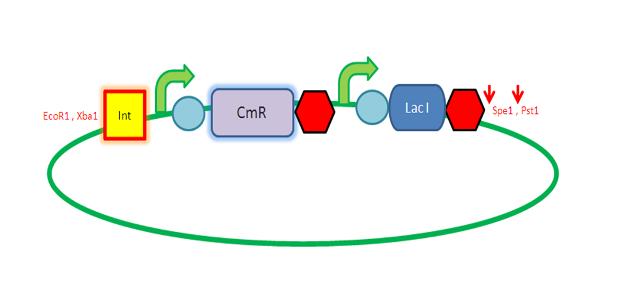
| AmyE Vector
|
|
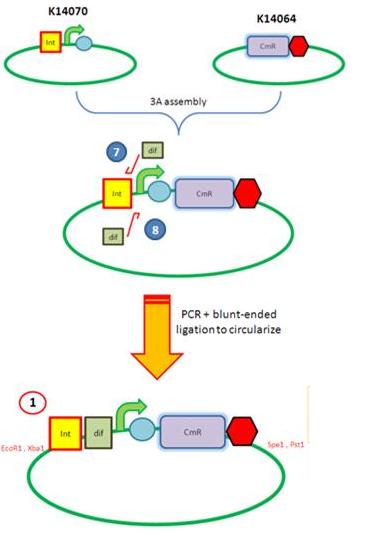
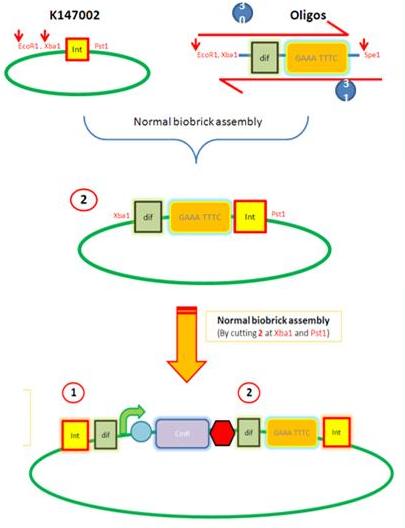
Starting from the top, we are assembling the first two fragments (K14070 and K14064) and K147002 with our oligos to add in a Dif site. Two dif sites on either side of resistance cassettes can be used to later excise antibiotic resistance from our final constructs.
K14070 and K14064 fragments
- DNA was taken out of the reigstry
- Cut with restriction enzymes
- Run on a gel to confirm correct cutting and estimate relative ratios of DNA for ligation
- Ligated overnight
- Transformed into E.Coli to Amplify the DNA
- Colony PCR has been used to confirm the correct insert size.
Next Steps:
- Extract the DNA with a miniprep
- Proceed to the next step - Reverse PCR
K14002 and oligos
- Ligated two single stranded oligos together to produce Dif and insertion site
- Standard biobrick assembly of oligos to K14002
- Ligation and transformation into E.coli competent cells (strain)
- Screen plated colonies for correct insertion
Next Steps:
- Purify the correct insert out of E.coli
- Next step assembly - LacI test vector and Final assembly vector
LacI Testing vector
Currently waiting for Part B step 2 Midi-prep results to start this step.
|
| Week 7
|
|
|
Day
|
Monday
|
Tuesday
|
Wednesday
|
Thursday
|
Friday
|
| Morning
|
- Starting assembly of AmyE vector:
- Restriction digestion of BB k14070 for the AmyE vector (using Eco and Spe)
- Restriction digestion of BB k14064 for the AmyE vector (using Eco and Xba)
- Restriction digestion of 5' integration site (k08) for the PyrD vector (using Eco and Spe)
- PCR amplification of vector backbone PSB1C3 for the PyrD vector
|
- Gel analysis and extraction of 5' int site for PyrD vector (repetition of step due to absence of DNA during gel analysis yesterday)
- Gel purification of k70
- Gel purification of k08
- Re-analysis of k70, k64 (for AmyE) on gel to work out ratios for ligation set up
- Re-analysis of k08, Pme oligos and pSB1C3 (for PyrD) on gel to work out ratios for ligation set up
- Restriction digestion of pveg promoter (k53) (using Eco and Spe) and spec cassette (k65) (using Xba and Pst) for PyrD vector
- Restriction digestion of pSB1C3 for PyrD (using Eco and Pst)
|
- Check for transformed colonies (colonies that have taken up the vector with the 5'diff) and prepare for colony PCR
- Gel purification of k53 and k65 for AmyE in preparation for ligations this afternoon
- Gel extraction and re-analysis on gel of k53, k65 and psB1C3 for Spec casette in preparation for ligations this afternoon
|
- Transformation of overnight ligations of: k64 and k70, k70 only, Spec and 5' PyrD diff
|
- Replica plating and colony PCR of: Spec and 5' PyrD diff
- Plate wash of: k64 and k70. k70 only was discarded since this was purely for a background check
|
| Afternoon
|
- PCR purification of PSB1C3 vector
- PCR purification of BB k14064 digestion products
- Gel analysis of digestion products of BB k14070
- Gel extraction of digestion products of BB k14070
|
- Restriction digestion of k64 and subsequent PCR purification (repetition of step due to absence of DNA during gel analysis)
- Gel analysis of k70, k64 (for AmyE) to work out ligation ratios
- Gel analysis and extraction of k53 and k65
- Bench (1 hour) and overnight ligation of 5'diff with the pSB1C3 vector
- Transformation of E.Coli with the bench ligated vector
|
- Dephosphorylation of k64 and set up of overnight ligations for k64 and k70 (vector and insert) and k64 (vector)only (for negative control; check of background)
- Set up overnight ligations of SpecR
|
|
- Gel analysis of colony PCR products of Spec and 5' PyrD diff
- Annealing of diff P oligos (used for both PyrD and AmyE vectors)
|
|
| Week 8
|
|
|
Day
|
Monday
|
Tuesday
|
Wednesday
|
Thursday
|
Friday
|
Saturday
|
| Morning
|
- Set up overnight ligations for standard assembly (BBA) and 3A cloning (3A) of dif P
- Restriction digestion of 3' integration site (K02) in the A2 vector ( Using Eco and Xba) for BBA
- Restriction digestion of 3' integration site (K09) in the AK3 vector ( Using Eco and Xba) for BBA
- PCR purification of 3' integration site (K02) in the A2 vector ( Using Eco and Xba) for BBA
- PCR purification of 3' integration site (K09) in the AK3 vector ( Using Eco and Xba) for BBA
- Set up 5 ml culture of spec from colony 1 of replica plate in shaking incubator @ 37 degrees
|
- Restriction digestion of 3' integration site (K02) in the A2 vector ( Using Xba and Pst) for 3A
- Restriction digestion of 3' integration site (K09) in the AK3 vector ( Using Xba and Pst) for 3A
|
- Transformations using the overnight ligations showed a lot of background. Therefore we set up ligations for K02 and K09 using 3A cloning. The results will show if this method is preferable due to less background.
- Midiprep of CmR vector and test digest using Eco and Spe
- Backbone PCR of PSB1C3 vector (1st attempt) for common use
|
- Replica plating of transformed colonies for k09 from the plate with the insert (diff P) - 45 sigle colonies were plated
- The first 15 of the above colonies were colony PCRed using dif PES Fwd and pSB Rev
|
- Gel analysis of colony PCR from yesterday (first 15 replica plated colonies
- Set up further colony PCR reactions for the same 15 colonies using pSB Fwd and pSB Rev primers
- Gel analysis of pSB1C3 - after backbone PCR and after digestion (looked contaminated!)
- Test digests of K54 K70 Midi prep using i) EcoRI, ii) SpeI and iii) EcoRI + SpeI
- Screen next 20 colonies by colony PCR, use higher temperatures to avoid previous non specific annealing
|
- Miniprep of 4 overnight cultures (dif P and 3' Insert for PyrD) - colonies 4,5,7 & 9
- Run Colony PCR results on a Gel - pick promissing candidates for mini prep.
|
| Afternoon
|
- Gel analysis of PCR purified K02 and K09 with the insert (diff P) in between to work out ratios for the liagation
- Dephosphorylation of digested A2 vector with 3' integration site (K02)
- Dephosphorylation of digested AK3 vector with 3' integration site (K09)
- Set up overnight ligation of A2 vector with 3' integration site (K02) with diff P (insert) and A2 vector only
- Set up overnight ligation of AK3 vector with 3' integration site (K09) with diff P (insert) and AK3 vector only
- Colony PCR and gel analysis of plated culture (from plate wash on Friday) with K64 and K70
- Overnight 100 ml culture of spec @ 14 degrees
|
- Electroporation of the 4 overnight ligations described on Thursday afternoon
- Gel extraction of the digestion products ( 3' integration sites - now our inserts) described this morning for 3A
- Gel analysis of gel extracted K02 nad K09 (inserts) with diff P (also an insert) and pSB1C3 (vector) in between
- Set up overnight 100 ml culture of CmR vector (K64 and K70) for midiprep tomorrow
- Set up overnight culture plates (AmpR) for the 4 electroporated cultures (colonies that survive will contain transformed cells
|
- PCR purification of PSB1C3 PCR amplified vector
- Check concentration of midi prepped CmR
- Run a gel to visualise the results - Gel contained pSB1C3 (PCR purified) , CmR (Midiprepped) and CmR digested (Midiprepped)
|
- Backbone PCR of pSB1C3 (2nd attempt), PCR purified and then digested with Eco and Pst
- Midipreps sent for sequencing ( Spec and CmR)
|
- Backbone PCR of pSB1C3 using PFU (3rd attempt)
- Set up overnight 5 ml cultures for miniprepping tomorrow - 4 cultures were set up by looking at the gel this morning; 2 positive looking (4 & 7), 1 negative (5) and one containing nothing (9)
|
- Diagnostic digests of minipreps - Two digests : One with Spe & Pst and other with Xba & Spe
|
|
| Week 9
|
|
|
Day
|
Monday
|
Tuesday
|
Wednesday
|
Thursday
|
Friday
|
| Morning
|
- Gel analysis of Mini preps and diagnostic digests from Saturday - Colony 4 looked best
- Test Digest of Mini Prep K02+dif EcoRI and SpeI
|
- Midiprep of Colony 4 - concentration 110 ng/ul
- Restriction digest of K02 from registrty for 3A assembly
|
- Gel purification of insert (35 ul)
- Gel analysis of vector and insert to work out ratios for ligations
|
- Transformation with overnight ligations
- PCR amplified the PSB1C3 vector for 3A assembly
- Gel Purified K02 and PBB1C3 digests
|
- The transformations were highly successful!! The '''Vector only''' plate showed no colonies and the '''Insert & Vector''' plate showed many individual colonies
- 10 individual colonies were replica plated and used for colony PCR
- Dephosphorylate PSB1C3 using alkaline phosphotase
|
| Afternoon
|
- Set up 200 ml overnight culture of colony 4 (containing Dif P and K09) for midiprep tomorrow
- Second Test digest SpeI PME - no positive results. Decided to repeat the step using 3A assembly method to reduce background from vector.
|
- Digests set up for Vector (SpecR) with Spe & Pst and Insert (dif P & K09)with Xba & Pst
- PCR purification of vetor (35 ul)
- Gel extraction of insert after gel analysis (35 ul)
- Midi prep of sample 8 K54 + K70
|
- Dephosphorylation of vector
- Set up two overnight ligations; Vector & Insert and Vector only
|
- Both ligations ('Insert & Vector' and 'Vector only') were plated in CmR and incubated overnight
|
- Gel analysis of colony PCR products
- Set up ligation reaction K02+dif using 3A method for Transformation on Monday.
|
|
| Week 13
|
|
|
Day
|
Monday
|
Tuesday
|
Wednesday
|
Thursday
|
Friday
|
| Morning
|
|
|
|
|
|
| Afternoon
|
|
|
|
|
|
|
|
 "
"