|
SEPTEMBER: WEEK 4
September, 27th
MiniPrep and E-P digest for I62-a, I62-b, I62-c, I62-d, I62-e, I62-f
DNA pcr for MyYeast 1-2-3
Gel run
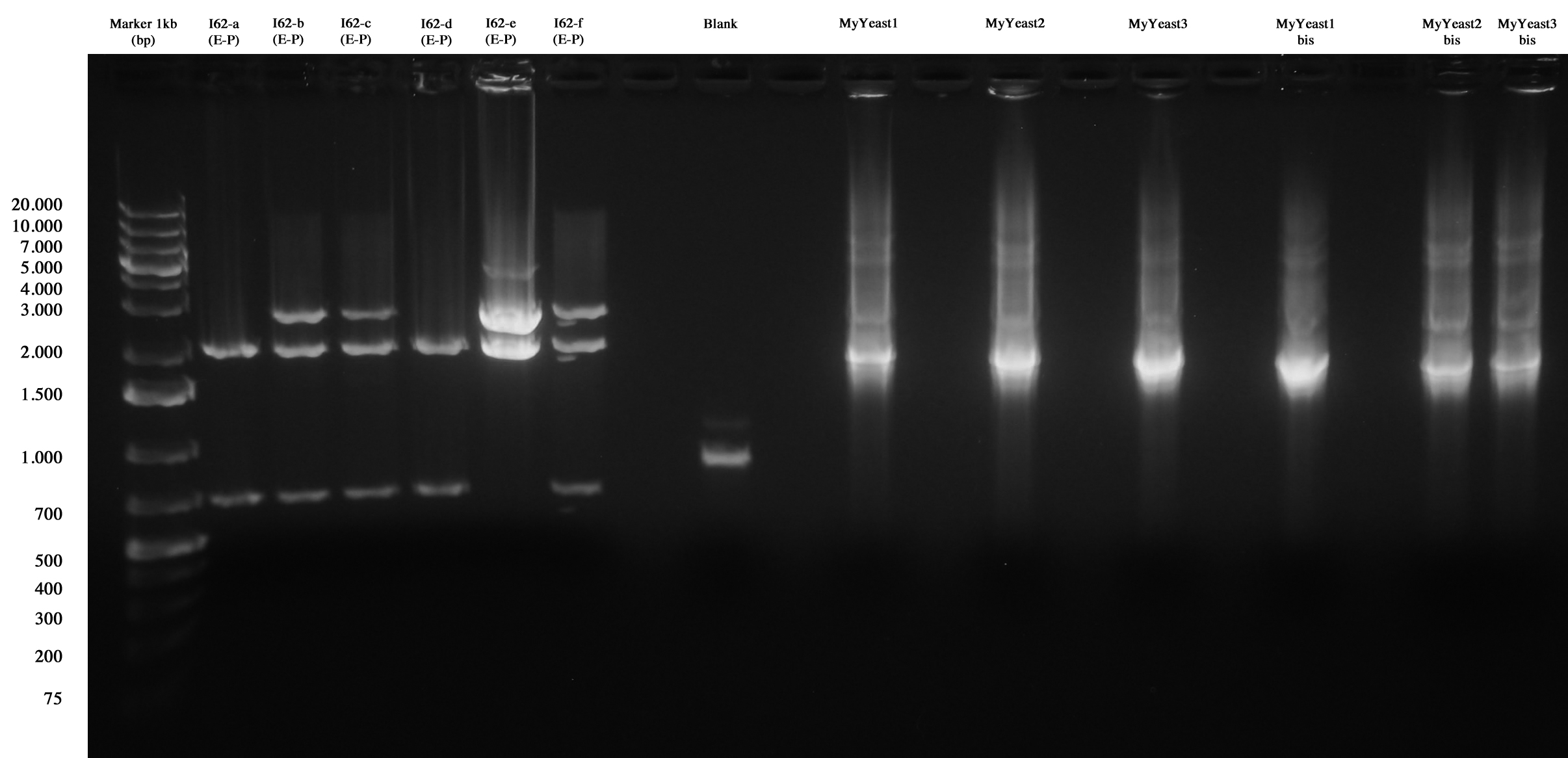 I62 and MyYeast series screening (I62, blank, MyYeastx,xbis).
I62-e was positive, so we made glycerol stock and stored it at -80°C.
Protein electrophoresis for phasins (Fede)
September, 28th
Tecan Test Giacomo
Miniprep and quantification for:
- I1: 59,6 ng/ul
- I4: 109,9 ng/ul
- I53: 160,3 ng/ul
- <partinfo>BBa_J04450</partinfo>-1:55,9 ng/ul
- <partinfo>BBa_J04450</partinfo>-2: 47 ng/ul
ON digestion of:
- I1 (E-S)
- I4 (E-S)
- I53 (E-X)
- <partinfo>BBa_J04450</partinfo>-1 (E-P)
- <partinfo>BBa_J04450</partinfo>-2 (E-P)
- MyYeast-3 (DNA already available) (E-S)
- <partinfo>pSB4C5</partinfo> (DNA already available) (E-P)
September, 29th
Tecan Test Giacomo
Colony PCR for 6 newly picked colonies.
 I55 colony PCR screening (blank blank 1 ..). No positives nor this time.
Gel run for all digested parts
 Digestions (4C5 myYeast3 I4 I53 J04450-2 J04450-1 I1). and gel run/cut/purification for:
- 4C5 (E-P): 19,3 ng/ul
- My Yeast 3 (E-S): 20,8 ng/ul
- I1: 3,8 ng/ul
- I4 (E-S): 14,2 ng/ul
- I53 (E-X): 21,6 ng/ul
- <partinfo>BBa_J04450</partinfo> (pLac-RBS-mRFP-TT) (E-P): 5,6 ng/ul
- <partinfo>pSB1C3</partinfo>: 10 ng/ul
Ligation of:
| I70=MyYeast 3(E-S)+I4(E-S)
|
| I71=MyYeast 3(E-S)+I1(E-S)
|
| I72=<partinfo>BBa_J04450</partinfo>(E-P)+<partinfo>pSB4C5</partinfo>
|
| I73= I38 (E-S) + I53 (E-X)
|
September, 30th
Tecan Test Nico
Transformation of I73 (1ul) into E. coli TOP10. It was plated on LB+Amp 100 ug/ml agar plate.
Trasformation of:
- I70 (myYeast with I4, Ptef1-mOrange-tAdh1) in TOP10 and plated on LB+Amp
- I71 (myYeast with I1, mOrange-tAdh1) in TOP10 and plated on LB+Amp
- I72 (pLac-RFP in pSB4C5) in TOP10 and plated on LB+Cm 12,5
All plates were incubated at 37°C ON
Since I55 was negative again we decided to ligate it again (probably we had a bad digest for vector - I20 - and a good one for insert - we used I38 in other ligations without problems). We diegested already miniprepped DNA for three hours with 1 ul of enzymes:
Gel run/cut/extraction
I20 E-X: ng/ul
We repeated I55 ligation using newly digested vector and already digested I38 (E-S)
I55: I38 (E-S) + I20 (E-X)
Inoculum into 5ml LB+Amp of:
- I31
- I35
- I37
- I57
- I60
- INTEIN
- 3x <partinfo>BBa_J04450</partinfo> (to take <partinfo>pSB1C3</partinfo>, so <partinfo>pSB1C3</partinfo> from now on)
Cultures were let grow ON at 37°C, 220 rpm.
Inoculum of E. coli TOP10 in 1,5 ml LB, grown ON at 37çC 220 rpm. Tomorrow we will prepare competent cells!!
October, 1st
This morning, we checked tranformed plates:
I72 showed NO colony!! :/ we will repeat this ligation next week
I70 and I71 showed colonies, 5 for each plate were picked and inoculated in 1ml LB+Amp to prepare glycerol stocks and for screening.
I73
Transformation of I55 into E. coli DH5-alpha. It was plated on LB+Amp 100 agar plate.
Miniprep and Nanodrop quantification for:
(they were also send sequencing)
- INTEIN
- <partinfo>pSB1C3</partinfo>:
- I37:
- I53:
Digest:
- <partinfo>pSB1C3</partinfo>: E-P
- <partinfo>pSB1C3</partinfo>: E-S
- <partinfo>pSB1C3</partinfo>: X-P
- INTEIN: E-P
- I60: E-S
- I31: S-P
- I35: X-P
- I37: E-X
- I57: E-X
Gel run/cut/extraction/quantification:
(foto)
They were stored at -20°C
ON ligation of:
- IXX: I31 (S-P) + I35 (X-P) (in vector <partinfo>pSB1A2</partinfo>)
- INTEIN_C3: INTEIN (E-P) + <partinfo>pSB1C3</partinfo> (E-P)
Pick and inoculum of I73 into 5 ml LB+Amp 100. It was let grow ON at 37°C, 220 rpm.
October, 2nd
|